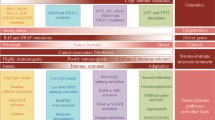
Key Points
-
The field of pharmacogenetics attempts to use genetic information to predict an individual's drug response. It is especially important in cancer chemotherapy given the narrow therapeutic index of these drugs.
-
So far, pharmacogenetic research has largely focused on the effect of single candidate polymorphisms. However, many of the genetic variants that are associated with extreme drug toxicity are rare and explain only a small portion of the variation seen in drug response.
-
Understanding the interactions of genetic variants within a biological or pharmacological pathway will allow for an improved ability to predict drug response.
-
Folate metabolism — a target of antifolate chemotherapeutic agents and thymidylate-synthase inhibitors — is a biological pathway of substantial interest to pharmacogenetic researchers.
-
Pharmacological pathways are being constructed for the systematic evaluation of the genes that regulate variation in the toxicity and efficacy of anticancer agents.
-
Mouse models show promise in identifying key enzymes in pharmacogenetic pathways and will allow study of genetic variation in these pathways.
Abstract
Inherited genetic variations can affect a patient's response to chemotherapeutic agents given for cancer. Pharmacogenetics aims to use knowledge of these variations to 'tailor' therapy for improved response and reduced toxicity. Most research so far has focused on single polymorphisms. A more comprehensive approach to predict treatment response will be to consider genetic variation in entire biological and pharmacological pathways. Of particular relevance to cancer chemotherapy is folate metabolism, which is the target of methotrexate and 5-fluorouracil. Furthermore, efforts have begun to construct pathways of genes that have pharmacological relevance for individual chemotherapeutic agents. Together, these pathway strategies offer a higher likelihood of achieving the promise of genetically guided cancer therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Relling, M. V. & Dervieux, T. Pharmacogenetics and cancer therapy. Nature Rev. Cancer 1, 99–108 (2001).
Ulrich, C. M., Robien, K. & Sparks, R. Pharmacogenetics and folate metabolism: a promising direction. Pharmacogenomics 3, 299–313 (2002).
Watters, J. W. & McLeod, H. L. Cancer pharmacogenomics: current and future applications. Biochim. Biophys. Acta 1603, 99–111 (2003).
Rogers, J. F., Nafziger, A. N. & Bertino, J. S. Jr. Pharmacogenetics affects dosing, efficacy, and toxicity of cytochrome P450-metabolized drugs. Am. J. Med. 113, 746–750 (2002).
Sindrup, S. H. & Brosen, K. The pharmacogenetics of codeine hypoalgesia. Pharmacogenetics 5, 335–346 (1995).
Relling, M. V. Mercaptopurine therapy intolerance and heterozygosity at the thiopurine S-methyltransferase gene locus. J. Natl Cancer Inst. 91, 2001–2008 (1999). Lowering the dose of mercaptopurine allowed children with the thiopurine S -methyltransferase enzyme deficiency to complete the course of chemotherapy and maintain sufficient erythrocyte thioguanine nucleotide concentrations.
Evans, W. E. Preponderance of thiopurine S-methyltransferase deficiency and heterozygosity among patients intolerant to mercaptopurine or azathioprine. J. Clin. Oncol. 19, 2293–2301 (2001).
McLeod, H. L. & Siva, C. The thiopurine S-methyltransferase gene locus: implications for clinical pharmacogenomics. Pharmacogenomics. 3, 89–98 (2002).
Milano, G. & McLeod, H. L. Can dihydropyrimidine dehydrogenase impact 5-fluorouracil-based treatment? Eur. J. Cancer 36, 37–42 (2000).
Mattison, L. K., Soong, R. & Diasio, R. B. Implications of dihydropyrimidine dehydrogenase on 5-fluorouracil pharmacogenetics and pharmacogenomics. Pharmacogenomics 3, 485–492 (2002).
Iyer, L. et al. Phenotype–genotype correlation of in vitro SN-38 (active metabolite of irinotecan) and bilirubin glucuronidation in human liver tissue with UGT1A1 promoter polymorphism. Clin. Pharmacol. Ther. 65, 576–582 (1999). Individuals who were homozygous for the UGT1A1 variant were found to have significantly lower levels of glucuronidation following irinotecan administration. grade III and grade IV diarrhoea and neutropaenia only occurred in patients who were UGT1A1 heterozygous or a homozygous variant.
Ando, Y. Polymorphisms of UDP-glucuronosyl transferase gene and irinotecan toxicity: a pharmacogenetic analysis. Cancer Res. 60, 6921–6926 (2000).
Iyer, L. UGT1A1*28 polymorphism as a determinant of irinotecan disposition and toxicity. Pharmacogenomics J. 2, 43–47 (2002).
Illmer, T. et al. MDR1 gene polymorphisms affect therapy outcome in acute myeloid leukemia patients. Cancer Res. 62, 4955–4962 (2002).
Park, D. J. A Xeroderma pigmentosum group D gene polymorphism predicts clinical outcome to platinum-based chemotherapy in patients with advanced colorectal cancer. Cancer Res. 61, 8654–8658 (2001).
Stoehlmacher, J. A polymorphism of the XRCC1 gene predicts for response to platinum based treatment in advanced colorectal cancer. Anticancer Res. 21, 3075–3079 (2001).
Marsh, S., Kwok, P. & McLeod, H. L. SNP databases and pharmacogenetics: great start, but a long way to go. Hum. Mutat. 20, 174–179 (2002).
Sachidanandam, R. A map of human genome sequence variation containing 1.42 million single nucleotide polymorphisms. Nature. 409, 928–933 (2001).
Wagner, C. Biochemical role of folate in cellular metabolism (ed. Bailey, L.) (Marcel Dekker, New York, 1995).
Longley, D. B., Harkin, D. P. & Johnston, P. G. 5-Fluorouracil: mechanisms of action and clinical strategies. Nature Rev. Cancer 3, 330–338 (2003).
Chu, E. & Allegra, C. in Cancer Chemotherapy and Biotherapy (eds. Chabner, B. & Longo, D.) 109–148 (Lippincott–Raven Publishers, Philadelphia, 1996).
Allegra, C. J. et al. Enhanced inhibition of thymidylate synthase by methotrexate polyglutamates. J. Biol. Chem. 260, 9720–9726 (1985).
Baggott, J. E., Vaughn, W. H. & Hudson, B. B. Inhibition of 5-aminoimidazole-4-carboxamide ribotide transformylase, adenosine deaminase and 5'-adenylate deaminase by polyglutamates of methotrexate and oxidized folates and by 5-aminoimidazole-4-carboxamide riboside and ribotide. Biochem. J. 236, 193–200 (1986).
Chu, E., Drake, J. C., Boarman, D., Baram, J. & Allegra, C. J. Mechanism of thymidylate synthase inhibition by methotrexate in human neoplastic cell lines and normal human myeloid progenitor cells. J. Biol. Chem. 265, 8470–8478 (1990).
Grem, J. in Cancer chemotherapy and biotherapy (eds. Chabner, B. & Longo, D.) 149–211 (Lippincott–Raven Publishers, Philadelphia, 1996).
McGavin, J. K. & Goa, K. L. Capecitabine: a review of its use in the treatment of advanced or metastatic colorectal cancer. Drugs 61, 2309–2326 (2001).
Blount, B. C. Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: implications for cancer and neuronal damage. Proc. Natl Acad.Sci. USA 94, 3290–3295 (1997).
Duthie, S. J. & McMillan, P. Uracil misincorporation in human DNA detected using single cell gel electrophoresis. Carcinogenesis 18, 1709–1714 (1997).
Duthie, S. J. & Hawdon, A. DNA instability (strand breakage, uracil misincorporation, and defective repair) is increased by folic acid depletion in human lymphocytes in vitro. FASEB J. 12, 1491–1497 (1998).
Lorico, A. Accumulation of DNA strand breaks in cells exposed to methotrexate or N10-propargyl-5,8-dideazafolic acid. Cancer Res. 48, 2036–2041 (1988).
Chabner, B. A. & Young, R. C. Threshold methotrexate concentration for in vivo inhibition of DNA synthesis in normal and tumorous target tissues. J. Clin. Invest. 52, 1804–1811 (1973).
Sirotnak, F. M. & Moccio, D. M. Pharmacokinetic basis for differences in methotrexate sensitivity of normal proliferative tissues in the mouse. Cancer Res. 40, 1230–1234 (1980).
Kaneda, S. Role in translation of a triple tandemly repeated sequence in the 5'-untranslated region of human thymidylate synthase mRNA. Nucleic Acids Res. 15, 1259–1270 (1987).
Marsh, S. Novel thymidylate synthase enhancer region alleles in African populations. Hum. Mutat. 16, 528 (2000).
Horie, N., Aiba, H., Oguro, K., Hojo, H. & Takeishi, K. Functional analysis and DNA polymorphism of the tandemly repeated sequences in the 5'-terminal regulatory region of the human gene for thymidylate synthase. Cell Struct. Funct. 20, 191–197 (1995).
Pullarkat, S. T. Thymidylate synthase gene polymorphism determines response and toxicity of 5-FU chemotherapy. Pharmacogenomics J. 1, 65–70 (2001).
Trinh, B. N., Ong, C. -N., Coetzee, G. A., Yu, M. C. & Laird, P. W. Thymidylate synthase: a novel genetic determinant of plasma homocysteine and folate levels. Hum. Genet. 111, 299–302 (2002).
Skibola, C. F. Polymorphisms in the thymidylate synthase and serine hydroxymethyltransferase genes and risk of adult acute lymphocytic leukemia. Blood 99, 3786–3791 (2002).
Ulrich, C. M., Bigler, J., Bostick, R., Fosdick, L. & Potter, J. D. Thymidylate synthase promoter polymorphism, interaction with folate intake, and risk of colorectal adenomas. Cancer Res. 62, 3361–3364 (2002).
Ulrich, C. Searching expressed sequence tag databases: discovery and confirmation of a common polymorphism in the thymidylate synthase gene. Cancer Epidemiol. Biomarkers Prev. 9, 1381–1385 (2000).
Lenz, H. -J. A 6 base-pair deletion in the 3′UTR of the thymidylate synthase (TS) gene predicts TS mRNA expression in colorectal tumors. A possible candidate gene for colorectal cancer risk. Proc. Am. Assoc. Cancer Res. 43, 660 (2002).
Frosst, P. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nature Genet. 10, 111–113 (1995).
Skibola, C. F. Polymorphisms in the methylenetetrahydrofolate reductase gene are associated with susceptibility to acute leukemia in adults. Proc. Natl Acad. Sci. USA 96, 12810–12815 (1999).
Wiemels, J. L. Methylenetetrahydrofolate reductase (MTHFR) polymorphisms and risk of molecularly defined subtypes of childhood acute leukemia. Proc. Natl Acad. Sci. USA 98, 4004–4009 (2001).
Ma, J. Methylenetetrahydrofolate reductase polymorphism, dietary interactions, and risk of colorectal cancer. Cancer Res. 57, 1098–1102 (1997).
Chen, J. A methylenetetrahydrofolate reductase polymorphism and the risk of colorectal cancer. Cancer Res. 56, 4862–4864 (1996).
Ulrich, C. M. Colorectal adenomas and the C677T MTHFR polymorphism: evidence for gene–environment interaction? Cancer Epidemiol. Biomarkers Prev. 8, 659–668 (1999).
Slattery, M. L., Potter, J. D., Samowitz, W., Schaffer, D. & Leppert, M. Methylenetetrahydrofolate reductase, diet, and risk of colon cancer. Cancer Epidemiol. Biomarkers Prev. 8, 513–518 (1999).
Le Marchand, L. et al. B-vitamin intake, metabolic genes, and colorectal cancer risk (United States). Cancer Causes Control 13, 239–248 (2002).
Shaw, G. M., Rozen, R., Finnell, R. H., Wasserman, C. R. & Lammer, E. J. Maternal vitamin use, genetic variation of infant methylenetetrahydrofolate reductase, and risk for spina bifida. Am. J. Epidemiol. 148, 30–37 (1998).
Ou, C. Y. et al. 5,10 Methylenetetrahydrofolate reductase genetic polymorphism as a risk factor for neural tube defects. Am. J. Med. Genet. 63, 610–614 (1996).
Kluijtmans, L. A. Thermolabile methylenetetrahydrofolate reductase in coronary artery disease. Circulation 96, 2573–2577 (1997).
van der Put, N. M. et al. A second common mutation in the methylenetetrahydrofolate reductase gene: an additional risk factor for neural-tube defects? Am. J. Hum. Genet. 62, 1044–1051 (1998).
Weisberg, I., Tran, P., Christensen, B., Sibani, S. & Rozen, R. A second genetic polymorphism in methylenetetrahydrofolate reductase (MTHFR) associated with decreased enzyme activity. Mol. Genet. Metab. 64, 169–172 (1998).
Keku, T. et al. 5,10-Methylenetetrahydrofolate reductase codon 677 and 1298 polymorphisms and colon cancer in African Americans and whites. Cancer Epidemiol. Biomarkers Prev. 11, 1611–1621 (2002).
Chango, A. A polymorphism (80G->A) in the reduced folate carrier gene and its associations with folate status and homocysteinemia. Mol. Genet. Metab. 70, 310–315 (2000).
Marsh, S., Collie-Duguid, E. S., Li, T., Liu, X. & McLeod, H. L. Ethnic variation in the thymidylate synthase enhancer region polymorphism among Caucasian and Asian populations. Genomics 58, 310–312 (1999).
Marsh, S., McKay, J. A., Cassidy, J. & McLeod, H. L. Polymorphism in the thymidylate synthase promoter enhancer region in colorectal cancer. Int. J. Oncol. 19, 383–386 (2001).
Iacopetta, B., Grieu, F., Joseph, D. & Elsaleh, H. A polymorphism in the enhancer region of the thymidylate synthase promoter influences the survival of colorectal cancer patients treated with 5-fluorouracil. Br. J. Cancer 85, 827–830 (2001).
Ueland, P. M., Hustad, S., Schneede, J., Refsum, H. & Vollset, S. E. Biological and clinical implications of the MTHFR C677T polymorphism. Trends Pharmacol. Sci. 22, 195–201 (2001).
Wisotzkey, J. K., Toman, J., Bell, T., Monk, J. S. & Jones, D. MTHFR (C677T) polymorphisms and stage III colon cancer: response to therapy. Mol. Diagn. 4, 95–99 (1999).
Ulrich, C. M. et al. Pharmacogenetics of methotrexate: toxicity among marrow transplantation patients varies with the methylenetetrahydrofolate reductase polymorphism. Blood 98, 231–234 (2001). Increased severity of oral mucositis and slower platelet recovery was observed among individuals with the homozygous variant MTHFR 677TT genotype compared with individuals with the wild-type ( MTHFR 677CC) or heterozygous ( MTHFR 677CT) genotype following haematopoietic-cell transplantation for chronic myelogenous leukaemia.
Storb, R. et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N. Engl. J. Med. 314, 729–735 (1986).
Chiusolo, P. Preponderance of methylenetetra-hydrofolate reductase C677T homozygosity among leukemia patients intolerant to methotrexate. Ann. Oncol. 13, 1915–1918 (2002).
Toffoli, G. Effect of methylenetetrahydrofolate reductase 677C→T polymorphism on toxicity and homocysteine plasma level after chronic methotrexate treatment of ovarian cancer patients. Int. J. Cancer 103, 294–299 (2003).
Toffoli, G., Veronesi, A., Boiocchi, M. & Crivellari, D. MTHFR gene polymorphism and severe toxicity during adjuvant treatment of early breast cancer with cyclophosphamide, methotrexate, and fluorouracil (CMF). Ann. Oncol. 11, 373–374 (2000).
Taub, J. W. Polymorphisms in methylenetetra-hydrofolate reductase and methotrexate sensitivity in childhood acute lymphoblastic leukemia. Leukemia 16, 764–765 (2002).
Krajinovic, M., Costea, I. & Chiasson, S. Polymorphism of the thymidylate synthase gene and outcome of acute lymphoblastic leukaemia. Lancet 359, 1033–1034 (2002).
Laverdiere, C., Chiasson, S., Costea, I., Moghrabi, A. & Krajinovic, M. Polymorphism G80A in the reduced folate carrier gene and its relationship to methotrexate plasma levels and outcome of childhood acute lymphoblastic leukemia. Blood 100, 3832–3834 (2002).
Cohen, V. Methylenetetrahydrofolate reductase polymorphism in advanced colorectal cancer: a novel genomic predictor of clinical response to fluoropyrimidine-based chemotherapy. Clin. Cancer Res. 9, 1611–1615 (2003).
Watters, J. W. & McLeod, H. L. Using genome-wide mapping in the mouse to identify genes that influence drug response. Trends Pharmacol. Sci. 24, 55–58 (2003).
Haston, C. K. Bleomycin hydrolase and a genetic locus within the MHC affect risk for pulmonary fibrosis in mice. Hum. Mol. Genet. 11, 1855–1863 (2002). This report outlines how mouse models were used to conduct a genome-wide screen for identification of susceptibility loci for bleomycin-induced pulmonary fibrosis.
Marton, M. J. Drug target validation and identification of secondary drug target effects using DNA microarrays. Nature Med. 4, 1293–1301 (1998).
van de Vijver, M. J. et al. A gene-expression signature as a predictor of survival in breast cancer. N. Engl. J. Med. 347, 1999–2009 (2002). This report outlines the use of microarray analysis to classify patients with breast cancer as having a gene-expression profile that is consistent with either poor or good prognosis. Multivariate Cox regression analysis found the gene-expression profile to be a strong predictor of disease outcome.
van't Veer, L. J., et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature 415, 530–536 (2002).
Rothenberg, M. L., Carbone, D. P. & Johnson, D. H. Improving the evaluation of new cancer treatments: challenges and opportunities. Nature Rev. Cancer 3, 303–309 (2003).
Villafranca, E. et al. Polymorphisms of the repeated sequences in the enhancer region of the thymidylate synthase gene promoter may predict downstaging after preoperative chemoradiation in rectal cancer. J. Clin. Oncol. 19, 1779–1786 (2001). Patients with rectal cancer receiving pre-operative 5-fluorouracil who were homozygous for the triple 28bp enhancer-region repeat of the thymidylate-synthase gene were found to have a lower probability of tumour downstaging and lower disease-free survival rates when compared with patients who were homozygous for the double repeat or heterozygous 2/3 repeat.
Park, D. J. et al. Thymidylate synthase gene polymorphism predicts response to capecitabine in advanced colorectal cancer. Int. J. Colorectal Dis. 17, 46–49 (2002).
Acknowledgements
Support for K. R. was provided by a training grant from the National Cancer Institute. H. M. is supported in part by the National Institute of Health Pharmacogenetics Research Network.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
Cancer.gov
LocusLink
FURTHER INFORMATION
Pharmacogenetics and Pharmacogenomics Knowledge Base
Pharmacogenetics Research Network, Washington University
Kyoto Encyclopedia of Genes and Genomes
Glossary
- THERAPEUTIC INDEX
-
The ratio of the median lethal dose to the median effective dose for a given medication. Used to describe the dose range over which a drug has a therapeutic effect without unacceptable toxicity.
- POLYMORPHISM
-
Variation within a gene (often at a single nucleotide) where two or more alleles exist at a frequency of at least 1% in the general population.
- FOLATE
-
One of the B vitamins. The primary role of this B vitamin is as a carrier of methyl groups, especially for purine, pyrimidine and methionine synthesis.
- PROBAND
-
An individual with the condition of interest, who serves as the starting point for exploration of a family pedigree for the genes that are responsible for the condition.
- CYTOCHROME-P450 ENZYMES
-
A family of haem-containing intracellular oxidizing enzymes that are responsible for the first phase of metabolism of many drugs and other ingested toxins.
- THYMIDYLATE-SYNTHASE INHIBITORS
-
A category of chemotherapeutic agents, including 5-fluorouracil, which tightly bind and inhibit the activity of thymidylate synthase, and therefore prevent DNA replication.
- MUCOSITIS
-
Inflammation, irritation and ulceration of the mucosal membranes, especially of the oral cavity and gastrointestinal tract, that occurs as a result of cytotoxic chemotherapy or radiation therapy.
- MYELOSUPPRESSION
-
Suppression of blood-cell formation in the bone marrow.
- TRANSCRIPTIONAL-ENHANCER ELEMENT
-
A region of DNA that might be several thousand base pairs upstream from a given gene's promoter region, but can bind with gene-regulatory proteins and increase the rate of transcription of that gene.
- LOSS OF HETEROZYGOSITY
-
The loss of one allele in a tumour cell for a gene in which the individual is normally heterozygous.
- HAZARD RATIO
-
The comparison of the risk of experiencing an event at any given point in time (hazard) among those with a particular risk factor with those without the risk factor.
- GRAFT-VERSUS-HOST DISEASE
-
A potentially life-threatening condition, which might occur following transplantation of solid organs or haematopoietic cells, in which donor T lymphocytes recognize host cells as foreign and attack host tissues.
- ODDS RATIO
-
A comparison of the risk of experiencing a particular outcome (such as a disease or adverse drug reaction) between two groups of people – one group with a particular risk factor (such as a genotype or a nutrient), and the other group without the risk factor being studied.
- TOPOISOMERASE I
-
An enzyme that creates a transient single-strand break in the phosphodiester backbone of DNA, allowing the strand of DNA to uncoil and rotate freely during replication.
Rights and permissions
About this article
Cite this article
Ulrich, C., Robien, K. & McLeod, H. Cancer pharmacogenetics: polymorphisms, pathways and beyond. Nat Rev Cancer 3, 912–920 (2003). https://doi.org/10.1038/nrc1233
Issue Date:
DOI: https://doi.org/10.1038/nrc1233
This article is cited by
-
Association of expression of epigenetic molecular factors with DNA methylation and sensitivity to chemotherapeutic agents in cancer cell lines
Clinical Epigenetics (2021)
-
Population pharmacokinetic model of irinotecan and its four main metabolites in patients treated with FOLFIRI or FOLFIRINOX regimen
Cancer Chemotherapy and Pharmacology (2021)
-
Influence of the ABCB1 polymorphisms on the response to Taxane-containing chemotherapy: a systematic review and meta-analysis
Cancer Chemotherapy and Pharmacology (2018)
-
Associations between polymorphisms in folate-metabolizing genes and pancreatic cancer risk in Japanese subjects
BMC Gastroenterology (2016)
-
Concerted changes in transcriptional regulation of genes involved in DNA methylation, demethylation, and folate-mediated one-carbon metabolism pathways in the NCI-60 cancer cell line panel in response to cancer drug treatment
Clinical Epigenetics (2016)