Key Points
-
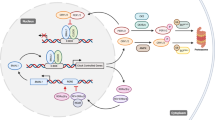
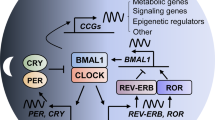
The circadian clock is the internal timing machine that can sustain rhythms of about 24 hours in the absence of external cues. The circadian clock is operated by the feedback loops of the circadian genes in the mammalian central pacemaker, as well as in most peripheral tissues.
-
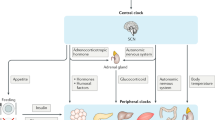
The mammalian central pacemaker is located in the suprachiasmatic nuclei (SCN) of the brain and controls the activity of peripheral clocks through the neuroendocrine and autonomic nervous systems. The circadian clock regulates hundreds of functions in the human body.
-
Disruption of circadian rhythms has been linked to mammalian tumorigenesis and tumour progression, and has been used as an independent prognostic factor of survival time for patients with certain metastatic cancers.
-
Normal and malignant tissues often show asynchronies in cell proliferation and metabolic rhythms. Based on these observations, cancer chronotherapy has been developed to improve the efficacy in cancer treatment and the quality of patients' life.
-
The circadian clock functions in vivo as a tumour suppressor at the systemic, cellular and molecular levels. The central clock is capable of generating 24-hour cell-proliferation rhythms in peripheral tissues through the activity of the neuroendocrine and autonomic nervous systems.
-
Molecular clocks in peripheral tissues control cell-proliferation rhythms by regulating the expression of cell-cycle genes. The core circadian genes are also involved in regulating cell proliferation. The circadian clock in peripheral tissues responds directly to DNA damage and could be important in the control of the cell cycle and apoptosis.
-
The molecular clockworks and cell-cycle clocks in peripheral tissues can be regulated simultaneously by the central clock, through interacting signalling pathways. Further study of the mechanism of the circadian clock in tumour suppression and the DNA-damage response has important implications for cancer therapy.
Abstract
The circadian rhythms are daily oscillations in various biological processes that are regulated by an endogenous clock. Disruption of these rhythms has been associated with cancer in humans. One of the cellular processes that is regulated by circadian rhythm is cell proliferation, which often shows asynchrony between normal and malignant tissues. This asynchrony highlights the importance of the circadian clock in tumour suppression in vivo and is one of the theoretical foundations for cancer chronotherapy. Investigation of the mechanisms by which the circadian clock controls cell proliferation and other cellular functions might lead to new therapeutic targets.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Pittendrigh, C. S. On temperature independence in the clock system controlling emergence time in Drosophila. Proc. Natl Acad. Sci. USA 40, 1018–1029 (1954).
Pittendrigh, C. S. Circadian systems. I. The driving oscillation and its assay in Drosophila pseudoobscura. Proc. Natl Acad. Sci. USA 58, 1762–1767 (1967).
Bargielle, T. A., Jackson, F. R. & Young, M. W. Restoration of circadian behavioral rhythms by gene transfer in Drosophila. Nature 312, 752–754 (1984).
Reddy, P. et al. Molecular analysis of the Period locus in Drosophila melanogaster and identification of a transcript involved in biological rhythms. Cell 38, 701–710 (1984).
Young, M. W. & Kay, S. A. Time zones: a comparative genetics of circadian clocks. Nature Rev. Genet. 2, 702–715 (2001).
Touitou, Y. & Haus, E. Biologic Rhythms in Clinical and Laboratory Medicine (eds Touitou Y. & Haus, E.) 188–207 (Springer-Verlag, Berlin, 1992).
Mormont, M. C. & Levi, F. Circadian-system alterations during cancer processes: a review. Int. J. Cancer 70, 241–247 (1997).
Roenneberg, T. & Lucas, R. J. Light, endocrine systems, and cancer — a view from circadian biologists. Neuroendocrinol. Lett. 23 (Suppl. 2), 82–83 (2002).
Schemhammer, E. S. et al. Rotating night shifts and risk of breast cancer in women participating in the nurses' health study. J. Natl Cancer Inst. 93, 1563–1568 (2001). Shows that disruption of circadian rhythm is involved in human cancer development.
Hansen, J. Increased breast cancer risk among women who work predominantly at night. Epidemiology 12, 74–77 (2001).
Fu, L., Pellicano, H., Liu, J., Hang, P. & Lee C. C. The circadian gene Period2 plays an important role in tumour suppression and DNA damage response in vivo. Cell 111, 41–50 (2002). First demonstration that loss of function in a mammalian circadian gene results in neoplastic growth and deficiencies in DNA-damage response.
Filipski, E. et al. Host circadian clock as a control point in tumour progression. J. Natl Cancer Inst. 94, 690–697 (2002).
Levi, F. From circadian rhythms to cancer chronotherapeutics. Chronobiol. Int. 19, 1–19 (2002).
King, D. P. et al. Positional cloning of the mouse circadian clock gene. Cell 89, 641–653 (1997).
Ralph, M. R. & Menaker, M. A mutation of the circadian system in gold hamsters. Science 241, 1225–1227 (1998).
Takano, A. et al. Cloning and characterization of rat casein kinase 1ε. FEBS Lett. 477, 106–112 (2000).
Hsu, D. S. et al. Putative human blue-light photoreceptors hCRY1 and hCRY2 are flavoproteins. Biochemistry 35, 13871–13877 (1996).
van der Spek, P. J. et al. Cloning, tissue expression, and mapping of a human photolyase homolog with similarity to plant blue-light receptors. Genomics 37, 177–182 (1996).
Thresher, R. J. et al. Role of mouse cryptochrome blue-light photoreceptor in circadian photoresponses. Science 282, 1490–1494 (1998.)
Tei, H. et al. Circadian oscillation of a mammalian homologue of the Drosophila period gene. Nature 389, 512–516 (1997).
Sun, Z. S. et al. RIGUI, a putative mammalian ortholog of the Drosophila period gene. Cell 90, 1003–1011 (1997). References 20 and 21 describe the identification of mammalian Period1 gene.
Shearman, L. P., Zylka, M. J., Weaver, D. R., Kolakowski, L. F. Jr & Reppert, S. M. Two period homologs: circadian expression and photo regulation in the suprachiasmatic nuclei. Neuron 19, 1261–1269 (1997).
Takumi, T. et al. A light-independent oscillatory gene mPer3 in mouse SCN and OVLT. EMBO J. 17, 4753–4759 (1998).
Albrecht, U., Sun, Z. S., Eichele, G. & Lee, C. C. A differential response of two putative mammalian circadian regulators, mPer1 and mPer2 to light. Cell 91, 1055–1064 (1997).
Hogenesch, J. B., Gu, Y. Z., Jain, S. & Bradfield, C. A. The basic–helix–loop–helix–PAS orphan MOP3 forms transcriptionally active complexes with circadian and hypoxia factors. Proc. Natl Acad. USA Sci. 95, 5474–5479 (1998).
Honma, S. et al. Circadian oscillation of BMAL1, a partner of a mammalian clock gene Clock, in rat suprachiasmatic nucleus. Biochem. Biophys. Res. Commun. 250, 83–87 (1998).
Reppert, S. M. & Weaver, D. R. Molecular analysis of mammalian circadian rhythms. Ann. Rev. Physiol. 63, 647–676 (2001).
Reppert, S. M. & Weaver, D. R. Coordination of circadian timing in mammals. Nature 418, 935–941 (2002).
Panda, S, Hogenesch, J. B. & Kay, S. A. Circadian rhythms from flies to human. Nature 417, 329–335 (2002). References 27–29 summarize the current understanding of the mammalian circadian clock.
Moore, R. Y. & Eichler, V. B. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesion in rat. Brain Res. 42, 201–206 (1972).
Stephan, F. K. & Zuker, I. Circadian rhythm in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesion. Proc. Natl Acad. Sci. USA 69, 1583–1586 (1972). References 30 and 31 show that the suprachiasmatic nucleus (SCN) in the brain is the master circadian clock in mammals.
Welsh, D. K., Logothelis, D. E., Meister, M. & Reppert, S. M. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phase circadian firing rhythms. Neuron 14, 697–706 (1995).
Liu, C., Weaver, D. R., Stogatz, S. H. & Reppert, S. M. Cellular construction of a circadian clock: period determination in the suprachiasmatic nucleus. Cell 91, 855–860 (1997).
Wright, K. P. Jr & Czeisler, C. A. Absence of circadian phase resetting in response to bright light behind the knee. Science 297, 571 (2002).
Lucas, R. et al. Identifying the photoreceptive inputs to the mammalian circadian system using transgenic and retinally degenerate mice. Behav. Brain Res. 125, 97–102 (2001).
Freedman, M. S. et al. Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science 284, 502–504 (1999).
Lucas, R. J., Freedman, M. S., Munoz, M., Garcia-Fernandez, J. M. & Foster, R. G. Regulation of the mammalian pineal by non-rod, non-cone, ocular photoreceptors. Science 284, 505–507 (1999).
Czeisler, C. A. et al. Suppression of melatonin secretion in some blind patients by exposure to bright light. N. Engl. J. Med. 332, 6–11 (1995).
Gooley, J. J., Lu, J., Chou, T. C., Scammell, T. E. & Saper, C. B. Melanopsin in cells of origin of the retinohypothalamic tract. Nature Neurosci. 4, 1165 (2001).
Hattar, S, Liao, H. W., Takao, M., Berson, D. M. & Yau, K. W. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science 295, 1065–1070 (2002).
Ruby, N. F. et al. Role of melanopsin in circadian responses to light. Science 298, 2211–2213 (2002).
Panda, S. et al. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science 298, 2213–2216 (2002).
Van Gelder, R. N., Wee, R., Lee, J. A. & Tu, D. C. Reduced pupillary light responses in mice lacking cryptochromes. Science 299, 222 (2002).
Bartness, T. J., Song, C. K. & Dernas, G. E. SCN efferents to peripheral tissues: implications for biological rhythms. J. Biol. Rhythms 16, 196–204 (2001).
Kalsbeek, A. & Buijs, R. M. Output pathways of the mammalian suprachiasmatic nucleus: coding circadian time by transmitter selection and specific targeting. Cell Tissue Res. 309, 109–118 (2002).
Buijs, R. M. et al. Anatomical and functional demonstration of a multisynaptic suprachiasmatic nucleus adrenal (cortex) pathway. Eur. J. Neurosci. 11, 1535–1544 (1999).
Zylka, M. J., Shearman, L. P., Weaver, D. R. & Reppert, S. M. Three period homologs in mammals: differential light responses in the suprachiasmatic circadian clock and oscillating transcripts outside of brain. Neuron 20, 1103–1110 (1998).
Morse, D., Cermakian, N., Brancorsini, S., Parvinen, M. & Sassone-Corsi, P. No circadian rhythms in testis: Period1 expression is clock independent and developmentally regulated in the mouse. Mol. Endocrinol. 17, 141–151 (2003).
Balsalobre, A., Damiola, F. & Schibler, U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell 93, 929–937 (1998).
Sakamoto, K. et al. Multitissue circadian expression of rat period homolog (rPer2) mRNA is governed by the mammalian circadian clock, the suprachiasmatic nucleus in the brain. J. Biol. Chem. 273, 27039–27042 (1998).
Damiola, F. et al. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 14, 2950–2961 (2000).
Stokkan, K. A., Yamazaki, S., Tei, H., Sakaki, Y. & Menaker, M. Entrainment of the circadian clock in the liver by feeding. Science 291, 490–493 (2001). References 51 and 52 show that the peripheral clocks can be entrained by non-photo stimuli, such as restricted feeding.
Rutter, J., Reick, M., Wu, L. C. & McKnight, S. L. Regulation of clock and NPAS2 DNA binding by the redox state of NAD cofactors. Science 293, 510–514 (2001).
Rutter, J., Reick, M. & McKnight, S. L. Metabolism and the control of circadian rhythms. Annu. Rev. Biochem. 71, 307–331 (2002).
Le Minh, N., Damiola, F., Tronche, F., Schutz, G. & Schibler, U. Glucocorticoid hormones inhibit food-induced phase-shifting of peripheral circadian oscillators. EMBO J. 20, 7128–2136 (2001).
Kornmann, B., Preitner, N., Rifat, D., Fleuury–Olela, F. & Schibler, U. Analysis of circadian liver gene expression by ADDER, a highly sensitive method for the display of differentially expressed mRNAs. Nucleic Acids Res. 29, E51 (2001).
Panda, S. et al. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 109, 307–320 (2002).
Duffield, G. E. et al. Circadian programs of transcriptional activation, signaling, and protein turnover revealed by microarray analysis of mammalian cells. Curr. Biol. 12, 551–557 (2002).
Akhtar, R. A. et al. Circadian cycling of the mouse liver transcriptome, as revealed by cDNA microarray, is driven by the suprachiasmatic nucleus. Curr. Biol. 12, 540–550 (2002).
Kita, Y. et al. Implications of circadian gene expression in kidney, liver and the effects of fasting on pharmacogenomic studies. Pharmacogenetics 12, 55–65 (2002).
Storch, K. F. et al. Extensive and divergent circadian gene expression in liver and heart. Nature 417, 78–83 (2002).
Jochle, W. Trends in photophysiologic concept. Ann. NY Acad. Sci. 117, 88–104 (1964).
Hamilton, T. Influence of environmental light and melatonin upon mammary tumour induction. Br. J. Surg. 56, 764–766 (1969).
Aubert. C., Janiaud, P. & Lecalvez, J. Effect of pinealectomy and melatoning on mammary tumour growth in Sprague Dawley rats under different conditions of lighting. J. Neural Trans. 47, 121–130 (1980).
Mhatre, M. C., Shan, P. N. & Juneja, H. S. Effect of varying photoperiods on mammary morphology, DNA synthesis, and hormone profile in female rats. J. Natl Cancer Inst. 72, 1411–1416 (1984).
Shah, P. N., Mhatre, M. C. & Kothari, L. S. Effect of melatonin on mammary carcinogenesis in intact and pinealectomized rats in varying photoperiods. Cancer Res. 44, 3403–3407 (1984).
Van den Heiligenberg, S. et al. The tumour promoting effect of constant light exposure on diethylnitrosamine-induced hepatocarcinogenesis in rats. Life Sci. 64, 2523–2334 (1999).
Davis, S., Mirick, D. K. & Stevens, R. G. Night shift work, light at night and risk of breast cancer. J. Natl Cancer Inst. 93, 1513–1515 (2001).
Rafneeon, V., Tulinius, H., Jonasson, J. G. & Hrafnkelsson, J. Risk of breast cancer in female flight attendants: a population-based study (Iceland). Cancer Causes Control 12, 95–101 (2001).
Keith, L. G., Oleszczuk, J. J. & Laguens, M. Circadian rhythm chaos: a new breast cancer marker. Int. J. Fertil. Womens Med. 46, 238–247 (2001).
Li, J. C. & Xu, F. Influence of light-dark shifting on immune system, tumour growth and life span of rats, mice and fruit flies as well as on the concentration of melatonin. Biol. Signals 6, 77–89 (1997).
Sephton, S. E., Sapolsky, R. M., Kraemer, H. C. & Spiegel, D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J. Natl Cancer Inst. 92, 994–1000 (2000).
Mormont, M. C. et al. Marked 24-h rest/activity rhythms are associated with better quality of life, better response, and longer survival in patients with metastatic colorectal cancer and good performance status. Clin. Cancer Res. 6, 3038–3045 (2000).
Wood, P. A. & Hrushesky, W. J. M. Circadian rhythms and cancer chemotherapy. Crit. Rev. Eukaryot. Gene Expr. 6, 299–343 (1996). Summary of chronotherapy for human cancer patients.
Bruguerolle, B. Chronopharmacokinetics: current status. Clin. Pharmacokinet. 35, 83–94 (1998).
Hori, K., Zhang, Q. H., Li, H. C., Saito, S. & Sato, Y. Timing of cancer chemotherapy based on circadian variations in tumour tissue blood flow. Int. J. Cancer. 65, 360–364 (1996).
Smaaland, R. et al. DNA sysnthesis in human bone marrow is circadian stage dependent. Blood 77, 2603–2611 (1991).
Bjarnason, G. A. & Jordan, R. Circadian variation of cell proliferation and cell cycle protein expression in man: clinical implications. Prog. Cell Cycle Res. 4,193–206 (2000).
Cooper, Z. K. & Schiff, A. Mitotic rhythm in human epidermis. Proc. Soc. Exp. Biol. Med. 39, 323–352 (1938).
Buchi, K. N. et al. Circadian rhythm of cellular proliferation in the human rectal mucosa. Gastroenterol. 101, 410–415 (1991).
Smaaland, R. Circadian rhythm of cell division. Prog. Cell Cycle Res. 2, 241–266 (1996).
Dublin, W. B., Gregg, R. O. & Broders, A. C. Mitosis in specimens removed during day and night from carcinoma of large intestine. Arch. Pathol. 30, 893–911 (1940).
Hrushesky, W. J. M., Lannin, D. & Haus, E. Evidence from an ontogenetic basis for circadian coordination of cancer proliferation. J. Natl Cancer Inst. 90, 1480–1484 (1998).
Izquierdo, J. N. Increased cell proliferation with persistence of circadian rhythms in hamster cheek pouch neoplasms. Cell Tissue Kinet. 10, 313–322 (1977).
Echave Llanos, J. M. & Nash, R. E. Mitotic circadian rhythm in a fast-growing and a slow-growing hepatoma: mitotic rhythm in hepatomas. J. Natl Cancer Inst. 44, 581–584 (1970).
Klevecz, R. R., Shymko, R. M., Blumenfeld, D. & Braly, P. S. Circadian gating of S phase in human ovarian cancer. Cancer Res. 47, 6267–6271 (1987).
Klevecs, R. R. & Braley, P. S. Circadian and ultradian cytokinetic rhythms of spontaneous human cancer. Ann. NY Acad. Sci. 618, 257–276 (1991).
Tahti, E. Studies of the effect of X-radiation on 24-hour variations in the mitotic activity in human malignant tumours. Acta Pathol. Microbiol. Scand. 117 (Suppl.), 1–61 (1956).
Smaaland, R., Lote, K., Sotteen, R. B. & Laerum, O. O. DNA synthesis and ploidy in non-Hodgkin's lymphomas demonstrate lntrapatient variation depending on circadian stage of cell sampling. Cancer Res. 53, 3129–3138 (1993).
Granda, T. G. & Levi, F. Tumour-based rhythms of anticancer efficacy in experimental models. Chronobiol. Int. 19, 21–41 (2002). Summary of chronotherapy studies using animal models.
Haus, E. et al. Increased tolerance of leukemic mice to arabinosylcytosine with schedule-adjusted to circadian system. Science 177, 80–82 (1972).
Halberg, F. et al. Toward a chronotherapy of neoplasia: tolerance of treatment depends upon host rhythms. Experientia 29, 909–934 (1973).
Boughattas, N. et al. Circadian rhythm in toxicities and tissue uptake of 1. 2-diamminocyclohexane(trans-1)oxalatoplatinum (II) in mice. Cancer Res. 49, 3362–2268 (1989).
Ohdo, S. et al. Cell cycle-dependent chronotoxicity of irinotecan hydrochloride in mice. J. Pharmacol. Exp. Ther. 283, 1383–1388 (1997).
Focan, C. Pharmaco-economic comparative evaluation of combination chronotherapy vs. standard chemotherapy for colorectal cancer. Chronobiol. Int. 19, 289–297 (2002).
Rich, T. A., Shelton, C. H., Kirichenko, A. & Straume, M. Chronomodulated chemotherapy and irradiation: an idea whose time has come? Chronobiol. Int. 19, 191–205 (2002).
Mormont, M. C. et al. Marker rhythms of circadian system function: a study of patients with metastatic coloretal cancer and good performance status. Chronobiol. Int. 19, 141–155 (2002).
Rivard, G. C., Infante-Rivard, C., Dress, M. F., Leclerc, J. M. & Champagne, J. Circadian time-dependent response of childhood lymphoblastic leukemia to chemotherapy: a long-term follow-up study of survival. Chronobiol. Int. 10, 201–204 (1993).
Buijs, R. M. & Kalsbeek, A. Hypothalamic integration of central and peripheral clocks. Nature Rev. Nerosci. 2, 521–526 (2001).
Costa, L. G. et al. Modulation of DNA synthesis by muscarinic cholinergic receptors. Growth Facors 18, 227–236 (2001).
Jones, M. A. & Marfurt, C. F. Sympathetic stimulation of corneal epithelial proliferation in wounded and nonwounded rat eyes. Invest. Ophthalmol. Vis. Sci. 37, 2535–2547 (1996).
Suzuki, S. et al. Circadian rhythm of leukocytes and lymphocyte subsets and its possible correlation with the function of autonomic nervous system. Clin. Exp. Immunol. 110, 500–508 (1997).
Okamoto, S, Ibaraki, K., Hayashi, S. & Saito, M. Ventromedial hypothalamus suppresses splenic lymphocyte activity through sympathetic innervation. Brain Res. 739, 308–313 (1996).
Maestroni, G. J. et al. Neural and endogenous catecholamines in bone marrow. Circadian association of norepinephrine with hematopoiesis? Exp. Hematol. 26, 1172–1177 (1998).
Kiba, T. The role of the autonomic nervous system in liver regeneration and apoptosis — recent developments. Digestion 66, 79–88 (2002).
Lavoie, C. et al. β1/β2-adrenergic receptor heterodimerization regulates β2-adrenergic receptor internalization and ERK signaling efficacy. J. Biol. Chem. 277, 35402–35410 (2002).
Moverara, S., Lindberg, M. K., Faergemann, J., Gustafsson, J. A. & Ohlsson, C. Estrogen receptor alpha, but not estrogen recepotr beta, is involved in the regulation of the hair follicle cycling as well as the thickness of epidermis in male mice. J. Invest. Dermatol. 119, 1053–1058 (2002).
Song, R. X. & Santen, R. J. Apoptotic action of estrogen. Apoptosis 8, 55–60 (2003).
Clarke, R., Dickson, R. B. & Lippman, M. E. Hormonal aspects of breast cancer. Crit. Rev. Oncol. Hematol. 12, 1–23 (1992).
Yamamoto, T. et al. Inhibition of murine osteosarcoma cell proliferation by glucocorticoid. Anticancer 22, 4151–4156 (2002).
Latta, K., Krieg, R. J., Carbajo-Perez, E., Carbajo, S. & Chan, J. C. Effects of deflazacort and cortisone on cellular proliferation in the rat thymus. Life Sci. 71, 1951–1960 (2002).
Abo, T. & Kawamura, T. Immunomodulation by the autonomic nervous system: therapeutic approach for cancer, collagen disease, and inflammatory bowel disease. Therap. Apheresis 6, 348–357 (2002).
Haus, E. & Smolensky, M. H. Biologic rhythms in the immue system. Chronobiol. Int. 16, 581–622 (1999).
Dunn, G. P., Bruce, A. T., Ikeda, H., Old, L. J. & Schreiber, R. D. Cancer immunoediting: from immunosurveillance to tumour escape. Nature Immunol. 3, 991–998 (2002).
Roberts, J. E. Light and immunomodulation. Ann. NY Acad. Sci. 917, 435–445 (2000).
Ohdo, S., Koyanagi, S., Suyama, H., Higuchi, S. & Aramaki, H. Changing the dosing schedule minimizes the disruptive effects of interferon on clock function. Nature Med. 7, 356–360 (2001).
Miller, D. B. & O'Callaghan, J. P. Neuroendocrine aspects of the response to stress. Metabolism 51, 5–10 (2002).
Besedovsky, H., Rey, A. D., Sorkin, E. & Dinarello, C. A. Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science 233, 652–654 (1986).
Bjarnason, G. A., Jordan, R. C. & Sothern, R. B. Circadian variation in the expression of cell-cycle proteins in human oral epithelium. Am. J. Pathol. 154, 613–622 (1999). Shows that the expression of cell-cycle regulators follows circadian oscillating patterns in normal human tissues.
Bjarnason, G. A. et al. Circadian expression of clock genes in human oral mucosa and skin: association with specific cell-cycle phases. Am. J. Pathol. 158, 1793–1801 (2001).
Gao, Z. H., Seeling, J. M., Hill, V., Yochum, A. & Virshup, D. M. Casein kinase I phosphorylates and destabilizes the beta-catenin degradation complex. Proc. Natl Acad. Sci. USA 99, 1182–1187 (2002).
Lee, E., Salic, A. & Kirschner, M. W. Physiological regulation of β-catenin stability by Tcf3 and CK1epsilon. J. Cell Biol. 154, 983–993 (2001).
Schwarz-Romond, T. et al. The ankyrin repeat protein diversin recruits casein kinase I epsilon to the beta-catenin degradation complex and acts in both canonical Wnt and Wnt/JNK signaling. Genes Dev. 16, 2073–2084 (2002). Shows that the circadian regulator CKIε directly regulates the Wnt-signalling pathway by controlling β-catenin signalling.
Van de Wetering, M., de Lau, W. & Clevers, H. Wnt signaling and lymphocyte development. Cell 109, S13–S19 (2002).
Morin, P. J. β-Catenin signaling and cancer. Bioessays 21, 1021–1030 (1999).
Crawford, H. C. et al. The PEA3 subfamily of Ets transcription factors synergizes with beta-catenin–LEF-1 to activate matrilysin transcription in intestinal tumours. Mol. Cell. Biol. 21, 1370–1383 (2001).
Mann, B. et al. Target genes of beta-catenin–T-cell-factor/lymphoid-enhancer-factor signaling in human colorectal carcinomas. Proc. Natl Acad. Sci. USA 96, 1603–1608 (1999).
Tetsu, O. & McCormick, F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398, 422–426 (1999).
Ahmed, Y., Hayashi, S., Levine, A. & Wieschaus, E. Regulation of armadillo by a Drosophila APC inhibits neuronal apoptosis during retinal development. Cell 93, 1171–1182 (1998).
Gat, U., DasGupta, R., Degenstein, L. & Fuchs, E. De novo hair follicle morphogenesis and hair tumours in mice expressing a truncated beta-catenin in skin. Cell 95, 605–614 (1998).
Martinek, S., Inonog, S., Manoukian, A. S. & Young, M. W. A role for the segment polarity gene shaggy/GSK-3 in the Drosophila circadian clock. Cell 105, 769–779 (2001).
Pelengaris, S., Khan, M. & Evan, G. I. Suppression of Myc-induced apoptosis in beta cells exposes multiple oncogenic properties of Myc and triggers carcinogenic progression. Cell 109, 321–334 (2002).
Breckenridge, D. C. & Shore, G. C. Regulation of apoptosis by E1A and Myc oncoproteins. Crit. Rev. Eukaryot. Gene Expr. 10, 273–280 (2000).
Blyth, K. et al. Synergy between a human c-myc transgene and p53 null genotype in murine thymic lymphomas: contrasting effects of homozygous and heterozygous p53 loss. Oncogene 10, 1717–1723 (1995).
Elson, A., Deng, C., Campos-Torres, J., Donehower, L. A. & Leder, P. The MMTV/c-myc transgene and p53 null alleles collaborate to induce T-cell lymphomas, but not mammary carcinomas in transgenic mice. Oncogene 11, 181–190 (1995).
Kawara, S. et al. Low-dose ultraviolet B rays alter the mRNA expression of the circadian clock genes in cultured human keratinocytes. J. Invest. Dermatol. 119, 1220–1223 (2002).
Sugano, S., Andronis, C., Green, R. M., Wang, Z. Y. & Tobin, E. M. Protein kinase CK2 interacts with and phosphorylates the Arabidopsis circadian clock-associated 1 protein. Proc. Natl Acad. Sci. USA 95, 11020–11025 (1998).
Sugano, S., Andronis, C., Ong, M. S., Green, R. M. & Tobin, E. M. The protein kinase CK2 is involved in regulation of circadian rhythms in Arabidopsis. Proc. Natl Acad. Sci. USA 96, 12362–12366 (1999).
Yang, Y., Cheng, P. & Liu, Y. Regulation of the Neurospora circadian clock by casein kinase II. Genes Dev. 16, 994–1006 (2002).
Lin, J. M. et al. A role for casein kinase 2alpha in the Drosophila circadian clock. Nature 420, 816–820 (2002). Shows that casein kinase 2 is involved in Drosophila circadian-clock control.
Pulverer, B. J. et al. Site-specific modulation of c-Myc cotransformation by residues phosphorylated in vivo. Oncogene 9, 59–70 (1994).
Channavajhala, P. & Seldin, D. C. Functional interaction of protein kinase CK2 and c-Myc in lymphomagenesis. Oncogene 21, 5280–5288 (2002).
Landesman-Bollag, E. et al. Protein kinase CK2: signaling and tumourigenesis in the mammary gland. Mol. Cell. Biochem. 227, 153–165 (2001).
Keller, D. M. et al. A DNA damage-induced p53 serine 392 kinase complex contains CK2, hSpt16, and SSRP1. Mol. Cell 7, 283–292 (2001).
Field, M. D. et al. Analysis of clock proteins in mouse SCN demonstrates phylogenetic divergence of the circadian clockwork and resetting mechanisms. Neuron 25, 437–447 (2000).
Shigeyoshi, Y. et al. Light-induced resetting of a mammalian circadian clock is associated with rapid induction of the mPer1 transcript. Cell 91, 1043–1053 (1997). Shows that light-induced Per1 expression is associated with phase resetting in the mammalian central clock.
Takeuchi, J., Shannon, W., Aronin, N. & Schwartz, W. J. Compositional changes of AP-1 DNA-binding proteins are regulated by light in a mammalian circadian clock. Neuron 11, 825–836 (1993).
Obrietan, K., Impey, S. & Storm, D. R. Light and circadian rhythmicity regulate MAP kinase activation in the suprachiasmatic nuclei. Nature Neurosci. 1, 693–700 (1998).
Ding, J. M., Faiman, L. E., Hurst, W. J., Kuriashkina, L. R. & Gillette, M. U. Resetting the biological clock: mediation of nocturnal CREB phosphorylation via light, glutamate, and nitric oxide. J. Neurosci. 17, 667–675 (1997).
Weber, E. T., Gannon, R. L., Michel, A. M., Gillette, M. U. & Rea, M. A. Nitric oxide synthase inhibitor blocks light-induced phase shifts of the circadian activity rhythm, but not c-fos expression in the suprachiasmatic nucleus of the Syrian hamster. Brain Res. 692, 137–142 (1995).
Ginty, D. D. et al. Regulation of CREB phosphorylation in the suprachiasmatic nucleus by light and a circadian clock. Science 260, 238–241 (1993).
Hurst, W. J., Mitchell, J. W. & Gillette, M. U. Synchronization and phase-resetting by glutamate of an immortalized SCN cell line. Biochem. Biophys. Res. Commun. 298, 133–143 (2002).
Balsalobre, A. et al. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 289, 2344–2347 (2000). Shows that the mammalian peripheral clocks are controlled by hormonal signals throughout the 24-hour period.
McNamara, P. et al. Regulation of CLOCK and MOP4 by nuclear hormone receptors in the vasculature: a humoral mechanism to reset a peripheral clock. Cell 105, 877–889 (2001).
Yamamoto, K. R. Steroid receptor regulated transcription of specific genes and gene networks. Ann. Rev. Genet. 19, 209–252 (1985).
Hida, A. et al. The human and mouse Period1 genes: five well-conserved E-box additively contribute to the enhancement of mPer1 transcription. Genomics 65, 224–233 (2000).
Akashi, M. & Nishida, E. Involvement of the MAP kinase cascade in resetting of the mammalian circadian clock. Genes Dev. 14, 645–649 (2000).
Balsalobre, A., Marcacci, L. & Schibler, U. Multiple signaling pathways elicit circadian gene expression in cultured Rat-1 fibroblasts. Curr. Biol. 10, 1291–1294 (2000). References 157 and 158 show that mammalian peripheral clocks are regulated by multiple signalling pathways.
Williams, J. A., Su, H., Bernards, A., Field J. & Sehgal, A. A circadian ouput mediated by NF1 and the Ras/MAPK pathway. Science 293, 2251–2256 (2001).
Ko, G. Y.-P., Ko, M. L. & Dryer, S. E. Circadian regulation of cGMP-gated cationic channels of chick retinal cones: Erk MAP kinase and Ca2+/calmodulin–dependent protein kinase II. Neuron 29, 255–266 (2001).
Johnson, G. L. & Lapadat, R. Mitogene-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298, 1911–1912 (2002).
Stork, P. J. & Schmitt, J. M. Crosstalk between cAMP and MAP kinase signaling in regulation of cell proliferation. Trends Cell Biol. 12, 258–266 (2002).
Vaudry, D., Stork, P. J., Lazarovici, P. & Eiden, L. E. Signaling pathways for PC12 cell differentiation: making the right connections. Science 296, 1648–1649 (2002).
Neves, S. R., Ram, P. T. & Ivengar, R. G protein pathways. Science 296, 1636–1639 (2002).
Brunton, V. G. et al. The protrusive phase and full development of integrin-dependent adhesions in colon epithelial cells require FAK- and ERK-mediated actin spike formation: deregulation in cancer cells. Neoplasia 3, 215–226 (2001).
Fribourg, A. F., Knudsen, K. E., Strobeck, M. W., Lindhorst, C. M. & Knudsen, E. S. Differential requirements for Ras and the retinoblastoma tumour suppressor protein in the androgen dependence of prostatic adenocarcinoma cells. Cell Growth Differ. 11, 361–372 (2000).
Aguirre Ghiso, J. A. et al. Deregulation of the signaling pathways controlling urokinase production. Its relationship with the invasive phenotype. Eur. J. Biochem. 263, 295–304 (1999).
De Bono, J. S. & Rowinsky, E. K. Therapeutics targeting signal transduction for patients with colorectal carcinoma. Br. Med. Bull. 64, 227–254 (2002).
Shaulian, E. & Karin, M. AP-1 as a regulator of cell life and death. Nature Cell Biol. 4, E131–E136 (2002).
Brown, J. R. et al. Fos family members induce cell cycle entry by activating cyclin D1. Mol. Cell. Biol. 18, 5609–5619 (1998).
Schreiber, M. et al. Control of cell cycle progression by c-Jun is p53 dependent. 13, 607–619 (1999).
Shaulian, E. et al. The mammalian UV response: c-Jun induction is required for exit from p53-imposed growth arrest. Cell 103, 897–907 (2000).
Passegue, E. & Wagner, E. F. JunB suppresses cell proliferation by transcriptional activation of p16 (INK4a) expression. EMBO J. 19, 2969–2979 (2000).
Honrado, G. I. et al. The circadian system of c-fos deficient mice. J. Comp. Physiol. 178, 563–570 (1996).
Wang, J. et al. Retinoid-induced G1 arrest and differentiation activation are associated with a switch to cyclin-dependent kinase-activating kinase hypophosphorylation of retinoic acid receptor alpha. J. Biol. Chem. 277, 43369–43376 (2002).
Toyota, M. et al. Peroxisome proliferator-activated receptor gamma reduces the growth rate of pancreatic cancer cells through the reduction of cyclin D1. Life Sci. 70, 1565–1575 (2002).
Spinella, M. J. et al. Retinoic acid promotes ubiquitination and proteolysis of cyclin D1 during induced tumour cell differentiation. J. Biol. Chem. 274, 22013–2208 (1999).
Lin, R. J., Sternsdrof, T., Tini, M. & Evans, R, M. Transcriptional regulation in acute promyelocytic leukemia. Oncogene 20, 7204–7215 (2001).
Ayroldi, E. et al. Glucocorticoid-induced leucine zipper inhibits the Raf-extracellular signal-regulated kinase pathway by binding to Raf-1. Mol. Cell. Biol. 22, 7929–7941 (2002).
Herrlich, P. Cross-talk between glucocorticoid receptor and AP-1. Oncogene 20, 2465–2475 (2001).
Liu, J. L., Papachristor, D. N. & Patel, Y. C. Glucocorticoids activate somatostatin gene transcription through co-operative interaction with the cyclic AMP signaling pathway. Biochem. J. 301, 863–869 (1994).
Qiu, J. et al. Rapid activation of ERK1/2 mitogen-activated protein kinase by corticosterone in PC12 cells. Biochem. Biophys. Res. Commun. 287, 1017–1021 (2001).
Rider, L. G., Hirasawa, N., Santini, F. & Beaven, M. A. Activation of the mitogen-activated protein kinase cascade is suppressed by low concentrations of dexamethasone in mast cells. J. Immunol. 157, 2374–2380 (1996).
Gonzalez, M. V. et al. Glucocorticoids antagonize AP-1 by inhibiting the activation/phosphorylation of JNK without affecting its subcellular distribution. J. Cell Biol. 150, 1199–1208 (2000).
Cidlowski, J. A. et al. The biochemistry and molecular biology of glucocorticoid-induced apoptosis in the immune system. Recent Prog. Horm. Res. 51, 457–490 (1996).
Eastman-Reks, S. B. & Vedeckis, W. V. Glucocorticoid inhibition of c-Myc, c-Myb and c-Ki-ras expression in a mouse lymphoma cell line. Cancer Res. 46, 2457–2462 (1986).
Rhee, K., Bresnahan, W., Hirai, A., Hirai, M. & Thompson, E. A. c-Myc and cyclin D3 (CcnD3) genes are independent targets for glucocorticoid inhibition of lymphoid cell proliferation. Cancer Res. 55, 4188–4195 (1995).
Silver, R. et al. A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomoter rhythms. Nature 382, 810–813 (1996).
Lehman, M. N. et al. Circadian rhythmicity restored by neural transplant. Immunocytochemical characterization of the graft and its integration with host brain. J. Neurosci. 7, 1626–1638 (1987).
Meyer-Bernstein, E. L. et al. Effects of supreachiasmatic transplants on circadian rhythms of neuroendocrine function in golden hamsters. Endocrinology 140, 207–218 (1999).
Van Esseveldt, K. E., Lehman, M. N. & Boer, G. J. The supreachiasmatic nucleus and the circadian time-keeping stsem revisited. Brain Res. Brain Res. Rev. 33, 34–77 (2000).
Kalsbeek, A., Buijs, R. M., Van Heerikhuize, J. J., Arts, M. & Van Der Woude, T. P. Vasopressin-containing neurons of the supreachiasmatic nuclei inhibit corticosterone release. Brain Res. 580, 62–67 (1992).
Preitner, N. et al. The orphan nuclear receptor REV-ERBα controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 110, 251–260 (2002).
Etchegaray, J., Lee, C., Wade, P. & Reppert, S. M. Rhythmic histone acetylation underlies transcription in the mammalian circadian clock. Nature 421, 177–182 (2002).
Moore–Ede, M., Sulzman, F. & Fuller, C. The Clocks That Time Us: Physiology of Circadian Timing System 448 (Harvard Univ. Press, Cambridge, Massachusetts, 1982).
Liu, C. et al. Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron 19, 91–102 (1997).
Toh, K. L. et al. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science 291, 1040–1043 (2001).
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
Cancer.gov
LocusLink
FURTHER INFORMATION
Glossary
- SUPRACHIASMATIC NUCLEI
-
(SCN). The mammalian master circadian clock. The SCN are small bilateral structures located next to the third ventricle and just above the optic chiasm in mammalian brain. Each SCN nucleus contains about 10,000 neurons that are synchronized to generate coordinated circadian outputs in vivo.
- PHASE SHIFT
-
The displacement of waveform in time. When a waveform is displaced by a complete wavelength, it is described as having a phase shift of 360 degrees. When a waveform is displaced by a half a wavelength, it is described as having a phase shift of 180 degrees.
- MELANOPSIN-EXPRESSING RETINAL GANGLION CELLS
-
A small subset of retinal ganglion cells that are intrinsically photosensitive and express the opsin-like protein melanopsin. These neurons project directly to the suprachiasmatic nucleus of the mammalian central circadian clock, as well as to the intergeniculate leaflet and the olivary pretectal nucleus in the brain. Mice that are deficient in melanopsin show attenuated responses to light stimuli.
- PINEALECTOMY
-
Ablation of the pineal gland. The pineal gland is a cone-shape gland that is located at the posterior end of the third ventricle in the brain. The pineal gland produces melatonin, a hormone that is important for regulating circadian rhythmicity in humans. The level of melatonin rises at night and falls during the day.
Rights and permissions
About this article
Cite this article
Fu, L., Lee, C. The circadian clock: pacemaker and tumour suppressor. Nat Rev Cancer 3, 350–361 (2003). https://doi.org/10.1038/nrc1072
Issue Date:
DOI: https://doi.org/10.1038/nrc1072
This article is cited by
-
Combined use of multiparametric high-content-screening and in vitro circadian reporter assays in neurotoxicity evaluation
Archives of Toxicology (2024)
-
Nuclear receptor modulators inhibit osteosarcoma cell proliferation and tumour growth by regulating the mTOR signaling pathway
Cell Death & Disease (2023)
-
A new border for circadian rhythm gene NFIL3 in diverse fields of cancer
Clinical and Translational Oncology (2023)
-
Clock genes are expressed in cementum and regulate the proliferation and mineralization of cementoblasts
In Vitro Cellular & Developmental Biology - Animal (2023)
-
A2M is a potential core gene in intrahepatic cholangiocarcinoma
BMC Cancer (2022)