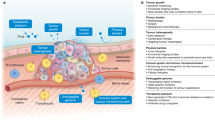
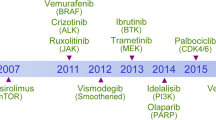
Abstract
There are, at present, ten times more anticancer drugs being tested in clinical trials than there were 15 years ago. Many of the new classes of agents, however, are predicted to work in only small subpopulations of patients, target unconventional aspects of tumour development and interact with other agents in an unpredictable manner. How can clinical trials be re-designed to accommodate the new features of targeted anticancer drugs?
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Pharmaceutical Research and Manufacturers Association. Pharmaceutical Industry Primer 2001: A Century of Progress [online], (cited 7 Mar 2003), <http://www.phrma.org/publications/publications//2001-07-09.508.pdf> (1991).
Burris, H. A. et al. Improvements in survival and clinical benefit with gemcitabine as front-line therapy for patients with advanced pancreas cancer: a randomized trial. J. Clin. Oncol. 15, 2403–2413 (1997).
Rothenberg, M. L. et al. A multicenter phase II trial of weekly irinotecan (CPT-11) in patients with previously treated colorectal cancer. Cancer 85, 786–795 (1999).
Pitot, H. C. et al. Phase II trial of irinotecan in patients with metastatic colorectal carcinoma. J. Clin. Oncol. 15, 2910–2919 (1997).
Rougier, P. et al. Randomised trial of irinotecan versus fluorouracil by continuous infusion after fluororuracil failure in patients with metastatic colorectal cancer. Lancet 352, 1407–1412 (1998).
Shepherd, F. A. et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J. Clin. Oncol. 18, 2095–2103 (2000).
Nabholtz, J. M. et al. Anastrozole is superior to tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: results of a North American multicenter randomized trial. Arimidex Study Group. J. Clin. Oncol. 18, 3758–3767 (2000).
Osborne, C. K. et al. Double-blind, randomized trial comparing the efficacy and tolerability of fulvestrant versus anastrozole in postmenopausal women with advanced breast cancer progressing on prior endocrine therapy: results of a North American trial. J. Clin. Oncol. 20, 3386–3395 (2002).
Buzdar, A. et al. Phase III, multicenter, double-blind, randomized study of letrozole, an aromatase inhibitor, for advanced breast cancer versus megestrol acetate. J. Clin. Oncol. 19, 3357–3366 (2001).
Saltz, L. et al. Cetuximab (IMC-C225) plus irinotecan (CPT-11) is active in CPT-11-refractory colorectal cancer that expresses epidermal growth factor receptor. Proc. Am. Soc. Clin. Oncol. 20, 3a (2001).
Herbst, R. S. et al. Selective oral epidermal growth factor receptor tyrosine kinase inhibitor ZD1839 is generally well tolerated and has activity in non-small-cell lung cancer and other solid tumors: results of a Phase I trial. J. Clin. Oncol. 20, 3815–3825 (2002).
Nelson, A. R., Fingleton, B., Rothenberg, M. L. & Matrisian, L. M. The matrix metalloproteinases: biologic activity and clinical implications. J. Clin. Oncol. 18, 1135–1149 (2000).
Kerbel, R. & Folkman, J. Clinical translation of angiogenesis inhibitors. Nature Rev. Cancer 2, 727–739 (2002).
Sirotnak, F. M., Zakowski, M. F., Miller, V. A., Scher, H. I. & Kris, M. G. Efficacy of cytotoxic agents against human tumor xenografts is markedly enhanced by coadministration of ZD1839 (Iressa), an inhibitor of EGFR tyrosine kinase. Clin. Cancer Res. 6, 4885–4892 (2000).
Wakeling, A. E. et al. ZD1839 (Iressa): an orally active inhibitor of epidermal growth factor signaling with potential for cancer therapy. Cancer Res. 62, 5749–5754 (2002).
Schuetz, J. D. et al. Potent BCRP inhibition by the ErbB1 inhibitor ZD1839 (Iressa) dramatically enhances oral bioavailability of topotecan and irinotecan in mice. Proc. Am. Assoc. Cancer Res. 43, 272 (2002).
Bramhall, S. R. et al. Marimastat as maintenance therapy for patients with advanced gastric cancer: a randomized trial. Br. J. Cancer 86, 1864–1870 (2002).
Ramaswamy, S., Ross, K. N., Lander, E. S. & Golub, T. R. A molecular signature of metastasis in primary solid tumors. Nature Genet. 33, 1–6 (2003).
Johnston, P. G. et al. Thymidylate synthase gene and protein expression are associated and correlate with response to 5-fluorouracil in human colorectal and gastric tumors. Cancer Res. 55, 1407–1412 (1995).
Betensky, R. A., Louis, D. N. & Cairncross, J. G. Influence of unrecognized molecular heterogeneity on randomized clinical trials. J. Clin. Oncol. 20, 2495–2499 (2002).
Buchdunger, E. et al. Abl protein-tyrosine kinase inhibitor STI571 inhibits in vitro signal transduction mediated by c-Kit and platelet-derived growth factor receptors. J. Pharmacol. Exp. Ther. 295, 139–145 (2000).
Demetri, G. D. et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N. Engl. J. Med. 347, 472–480 (2002).
Salonga, D. et al. Colorectal tumors responding to 5-fluorouracil have low gene expression levels of dihydropyrimidine dehydrogenase, thymidylate synthase, and thymidine phosphorylase. Clin. Cancer Res. 6, 1322–1327 (2000).
Relling, M. V. et al. Mercaptopurine therapy intolerance and heterozygosity at the thiopurine S-methyltransferase gene locus. J. Natl Cancer Inst. 91, 2001–2008 (1999).
Ino, Y. et al. Molecular subtypes of anaplastic oligodendroglioma: implications for patient management at diagnosis. Clin. Cancer Res. 7, 839–845 (2001).
Bramhall, S. R., Rosemurgy, A., Brown, P. D., Bowry, C. & Buckels, J. A. C. Marimastat as first-line therapy for patients with unresectable pancreatic cancer: a randomized trial. J. Clin. Oncol. 19, 3447–3455 (2001).
Coussens, L. M., Fingleton, B. & Matrisian, L. M. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science 295, 2387–2392 (2002).
Shepherd, F. A. et al. Prospective, randomized, double-blind, placebo-controlled trial of marimastat after response to first-line chemotherapy in patients with small-cell lung cancer: a trial of the National Cancer Institute of Canada Clinical Trials Group and the European Organization for Research and Treatment of Cancer. J. Clin. Oncol. 20, 4434–4439 (2002).
Bramhall, S. R. et al. A double-blind placebo-controlled, randomized study comparing gemcitabine and marimastat with gemcitabine and placebo as first line therapy in patients with advanced pancreatic cancer. Br. J. Cancer 87, 161–167 (2002).
Sparano, J. A. et al. Randomized Phase III trial of marimastat versus placebo in patients with metastatic breast cancer who have responding or stable disease after first-line chemotherapy: an Eastern Cooperative Oncology Group trial (E2196). Proc. Am. Soc. Clin. Oncol. 21, 44a (abst 173) (2002).
King, J., Clingan, P. & Morris, D. L. Placebo control double-blind randomised clinical trial of the matrix metalloproteinase inhibitor marimastat in patients with inoperable colorectal cancer liver metastases: significant survival advantage in patients with musculoskeletal symptoms. Proc. Am. Soc. Clin. Oncol. 21: 135a (abst 537) (2002).
British Biotech PLC. Results of Marimastat Study 131 in Patients with Glioblastoma [online], (cited 7 Mar 2003), <www.britbio.com/news/131.pdf> (2000).
Bissett, D. et al. Phase III study of the matrix metalloprotease inhibitor prinomastat in combination with gemcitabine and cisplatin in non-small cell lung cancer. Proc. Am. Soc. Clin. Oncol. 21, 296a (abst 1183) (2002).
Smylie, M. et al. Phase III study of the matrix metalloprotease inhibitor prinomastat in patients having advanced non-small cell lung cancer. Proc. Am. Soc. Clin. Oncol. 20, 307a (abst 1226) (2001).
Ahmann, F. R. et al. Interim results of a Phase III study of the matrix metalloprotease inhibitor prinomastat in patients having metastatic, hormone-refractory prostate cancer. Proc. Am. Soc. Clin. Oncol. 20L, 174a (abst 692) (2001).
Moore, M. J. et al. A comparison between gemcitabine and the matrix metalloproteinase inhibitor BAY 12–9566 in patients with advanced pancreatic cancer. Proc. Am. Soc. Clin. Oncol. 19, 240a (abst 930) (2000).
Bayer Pharmaceutical Division. Bayer Halts Clinical Trials Evaluating MMPI [online], (cited 7 Mar 2003), <www.bayerusa.com/news/co0221.asp> (1999).
Johnson, D. H. et al. ZD1839 (Iressa) in combination with paclitaxel and carboplatin in chemotherapy-naïve patients with advanced non-small-cell lung cancer: results from a Phase III clinical trial (INTACT 2). Ann. Oncol. 13 (Suppl. 5), 127 (abst 4680) (2002).
Giaccone, G. et al. A Phase III trial of ZD 1839 (Iressa) in combination with gemcitabine and cisplatin in chemotherapy-naïve patients with non-small-cell lung cancer (INTACT1). Ann. Oncol. 13 (Suppl. 5), 2 (abst 40) (2002).
van Cutsem, E. et al. Phase III trial comparing gemcitabine + R115777 (Zarnestra) versus gemcitabine + placebo in advanced pancreatic cancer. Proc. Am. Soc. Clin. Oncol. 21, 130a (abst 517) (2002).
Genentech. Phase III Trial with Avastin in Relapsed Breast Cancer Does Not Meet Primary Endpoint [online], (cited 7 Mar 2003), <www.genentech.com/gene/news/press-releases/detail.jsp?detail=5427> (2002).
SUGEN. Letter to SU5416 Investigators, February, 2002.
Acknowledgements
Supported, in part, by National Institutes of Health grants and the Ingram Charitable Trust.
Author information
Authors and Affiliations
Corresponding author
Related links
Rights and permissions
About this article
Cite this article
Rothenberg, M., Carbone, D. & Johnson, D. Improving the evaluation of new cancer treatments: challenges and opportunities. Nat Rev Cancer 3, 303–309 (2003). https://doi.org/10.1038/nrc1047
Issue Date:
DOI: https://doi.org/10.1038/nrc1047
This article is cited by
-
Innovative trial approaches in immune-mediated inflammatory diseases: current use and future potential
BMC Rheumatology (2021)
-
Nanoparticle core stability and surface functionalization drive the mTOR signaling pathway in hepatocellular cell lines
Scientific Reports (2017)
-
Targeted doxorubicin delivery based on avidin-biotin technology in cervical tumor cells
Macromolecular Research (2017)
-
Subgroup identification for treatment selection in biomarker adaptive design
BMC Medical Research Methodology (2015)
-
Modified response evaluation criteria in solid tumors is superior to response evaluation criteria in solid tumors for assessment of responses to sorafenib in patients with advanced hepatocellular carcinoma
BMC Research Notes (2015)