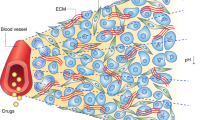
Abstract
Solid tumours need a blood supply, and a large body of evidence has previously suggested that they can grow only if they induce the development of new blood vessels, a process known as tumour angiogenesis. On the basis of this hypothesis, it was proposed that anti-angiogenic drugs should be able to suppress the growth of all solid tumours. However, clinical experience with anti-angiogenic agents has shown that this is not always the case. Reports of tumours growing without the formation of new vessels can be found in the literature dating back to the 1800s, yet no formal recognition, description and demonstration of their special biological status was made until recently. In 1996, we formally recognized and described non-angiogenic tumours in lungs where the only blood vessels present were those originating from normal lung tissue. This is far from an isolated scenario, as non-angiogenic tumour growth has now been observed in tumours of many different organs in both humans and preclinical animal models. In this Opinion article, we summarize how these tumours were discovered and discuss what we know so far about their biology and the potential implications of this knowledge for cancer treatment.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Medawar, P. B. Advice to a Young Scientist 73 (Basic Books, Persus Book Group, 1979).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Natale, G., Bocci, G. & Lenzi, P. Looking for the word “angiogenesis” in the history of health sciences: from ancient times to the first decades of the twentieth century. World J. Surg. 41, 1625–1634 (2017).
Folkman, J. Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 285, 1182–1186 (1971).
Dome, B., Hendrix, M. J., Paku, S., Tovari, J. & Timar, J. Alternative vascularization mechanisms in cancer: pathology and therapeutic implications. Am. J. Pathol. 170, 1–15 (2007).
Donnem, T. et al. Vessel co-option in primary human tumors and metastases: an obstacle to effective anti-angiogenic treatment? Cancer Med. 2, 427–436 (2013).
Gianni-Barrera, R., Trani, M., Reginato, S. & Banfi, A. To sprout or to split? VEGF, Notch and vascular morphogenesis. Biochem. Soc. Trans. 39, 1644–1648 (2011).
Nolan, D. J. et al. Bone marrow-derived endothelial progenitor cells are a major determinant of nascent tumor neovascularization. Genes Dev. 21, 1546–1558 (2007).
Albini, A., Tosetti, F., Li, V. W., Noonan, D. M. & Li, W. W. Cancer prevention by targeting angiogenesis. Nat. Rev. Clin. Oncol. 9, 498–509 (2012).
Jayson, G. C., Kerbel, R., Ellis, L. M. & Harris, A. L. Antiangiogenic therapy in oncology: current status and future directions. Lancet 388, 518–529 (2016).
Kerbel, R. S. Tumor angiogenesis. N. Engl. J. Med. 358, 2039–2049 (2008).
Weis, S. M. & Cheresh, D. A. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat. Med. 17, 1359–1370 (2011).
Harris, A. L. Hypoxia—a key regulatory factor in tumour growth. Nat. Rev. Cancer 2, 38–47 (2002).
Bridgeman, V. L. et al. Vessel co-option is common in human lung metastases and mediates resistance to anti-angiogenic therapy in preclinical lung metastasis models. J. Pathol. 241, 362–374 (2017).
Frentzas, S. et al. Vessel co-option mediates resistance to anti-angiogenic therapy in liver metastases. Nat. Med. 22, 1294–1302 (2016).
Breast Cancer Progression Working Party. Evidence for novel non-angiogenic pathway in breast-cancer metastasis. Lancet 355, 1787–1788 (2000).
Kusters, B. et al. Vascular endothelial growth factor-A(165) induces progression of melanoma brain metastases without induction of sprouting angiogenesis. Cancer Res. 62, 341–345 (2002).
Naresh, K. N., Nerurkar, A. Y. & Borges, A. M. Angiogenesis is redundant for tumour growth in lymph node metastases. Histopathology 38, 466–470 (2001).
Vermeulen, P. B. et al. Liver metastases from colorectal adenocarcinomas grow in three patterns with different angiogenesis and desmoplasia. J. Pathol. 195, 336–342 (2001).
Pezzella, F. et al. Angiogenesis in primary lung cancer and lung secondaries. Eur. J. Cancer 32A, 2494–2500 (1996).
Pezzella, F. et al. Non-small-cell lung carcinoma tumor growth without morphological evidence of neo-angiogenesis. Am. J. Pathol. 151, 1417–1423 (1997).
Seaman, S. et al. Eradication of tumors through simultaneous ablation of CD276/B7-H3-positive tumor cells and tumor vasculature. Cancer Cell 31, 501–515 (2017).
de Groot, J. F. et al. Tumor invasion after treatment of glioblastoma with bevacizumab: radiographic and pathologic correlation in humans and mice. Neuro. Oncol. 12, 233–242 (2010).
Jeong, H. S. et al. Investigation of the lack of angiogenesis in the formation of lymph node metastases. J. Natl. Cancer Inst. 107, djv155 (2015).
Keunen, O. et al. Anti-VEGF treatment reduces blood supply and increases tumor cell invasion in glioblastoma. Proc. Natl Acad. Sci. USA 108, 3749–3754 (2011).
Khan, K. A. & Kerbel, R. S. A. CD276 antibody guided missile with one warhead and two targets: the tumor and its vasculature. Cancer Cell 31, 469–471 (2017).
Kuczynski, E. A. et al. Co-option of liver vessels and not sprouting angiogenesis drives acquired sorafenib resistance in hepatocellular carcinoma. J. Natl. Cancer Inst. 108, djw030 (2016).
Leenders, W. P. et al. Antiangiogenic therapy of cerebral melanoma metastases results in sustained tumor progression via vessel co-option. Clin. Cancer Res. 10, 6222–6230 (2004).
Pezzella, F. & Gatter, K. C. Evidence showing that tumors can grow without angiogenesis and can switch between angiogenic and nonangiogenic phenotypes. J. Natl. Cancer Inst. 108, djw032 (2016).
Baker, G. J. et al. Mechanisms of glioma formation: iterative perivascular glioma growth and invasion leads to tumor progression, VEGF-independent vascularization, and resistance to antiangiogenic therapy. Neoplasia 16, 543–561 (2014).
Wesseling, P., van der Laak, J. A., de, L. H., Ruiter, D. J. & Burger, P. C. Quantitative immunohistological analysis of the microvasculature in untreated human glioblastoma multiforme. Computer-assisted image analysis of whole-tumor sections. J. Neurosurg. 81, 902–909 (1994).
Ritchie, A. C. in The Classification, Morphology, and Behaviour of Tumours in General Pathology (ed. Florey, H.) 551–597 (Lloyd-Luke, London, 1962).
Folkman, J. What is the evidence that tumors are angiogenesis dependent? J. Natl Cancer Inst. 82, 4–6 (1990).
Gimbrone, M. A. Jr., Leapman, S. B., Cotran, R. S. & Folkman, J. Tumor dormancy in vivo by prevention of neovascularization. J. Exp. Med. 136, 261–276 (1972).
Ide, A. G., Baker, N. H. & Warren, S. L. Vascularization of the Brown Pearce rabbit epithelioma transplant as seen in the transparent ear chamber. Am. J. Roentgenol. 42, 891–899 (1939).
Passalidou, E. et al. Vascular phenotype in angiogenic and non-angiogenic lung non-small cell carcinomas. Br. J. Cancer 86, 244–249 (2002).
Adighibe, O. et al. Is nonangiogenesis a novel pathway for cancer progression? A study using 3-dimensional tumour reconstructions. Br. J. Cancer 94, 1176–1179 (2006).
Stessels, F. et al. Breast adenocarcinoma liver metastases, in contrast to colorectal cancer liver metastases, display a non-angiogenic growth pattern that preserves the stroma and lacks hypoxia. Br. J. Cancer 90, 1429–1436 (2004).
Van den Eynden, G. G. et al. The histological growth pattern of colorectal cancer liver metastases has prognostic value. Clin. Exp. Metastasis 29, 541–549 (2012).
Kunkel, P. et al. Inhibition of glioma angiogenesis and growth in vivo by systemic treatment with a monoclonal antibody against vascular endothelial growth factor receptor-2. Cancer Res. 61, 6624–6628 (2001).
Berghoff, A. S. et al. Invasion patterns in brain metastases of solid cancers. Neuro. Oncol. 15, 1664–1672 (2013).
Bernsen, H. et al. Gliomatosis cerebri: quantitative proof of vessel recruitment by cooptation instead of angiogenesis. J. Neurosurg. 103, 702–706 (2005).
Carbonell, W. S., Ansorge, O., Sibson, N. & Muschel, R. The vascular basement membrane as “soil” in brain metastasis. PLoS ONE 4, e5857 (2009).
Hung, T. et al. Angiotropism in primary cutaneous melanoma with brain metastasis: a study of 20 cases. Am. J. Dermatopathol. 35, 650–654 (2013).
Siam, L. et al. The metastatic infiltration at the metastasis/brain parenchyma-interface is very heterogeneous and has a significant impact on survival in a prospective study. Oncotarget 6, 29254–29267 (2015).
Vermeulen, P. B., Sardari, N. P., Colpaert, C., Dirix, L. Y. & Van, M. E. Lack of angiogenesis in lymph node metastases of carcinomas is growth pattern-dependent. Histopathology 40, 105–107 (2002).
Goel, S. et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiol. Rev. 91, 1071–1121 (2011).
Auf, G. et al. Inositol-requiring enzyme 1alpha is a key regulator of angiogenesis and invasion in malignant glioma. Proc. Natl Acad. Sci. USA 107, 15553–15558 (2010).
Bentolila, L. A. et al. Imaging of angiotropism/vascular co-option in a murine model of brain melanoma: implications for melanoma progression along extravascular pathways. Sci. Rep. 6, 23834 (2016).
Boult, J. K. et al. Investigating intracranial tumour growth patterns with multiparametric MRI incorporating Gd-DTPA and USPIO-enhanced imaging. NMR Biomed. 29, 1608–1617 (2016).
Budde, M. D., Gold, E., Jordan, E. K., Smith-Brown, M. & Frank, J. A. Phase contrast MRI is an early marker of micrometastatic breast cancer development in the rat brain. NMR Biomed. 25, 726–736 (2012).
Budde, M. D., Gold, E., Jordan, E. K. & Frank, J. A. Differential microstructure and physiology of brain and bone metastases in a rat breast cancer model by diffusion and dynamic contrast enhanced MRI. Clin. Exp. Metastasis 29, 51–62 (2012).
Caspani, E. M., Crossley, P. H., Redondo-Garcia, C. & Martinez, S. Glioblastoma: a pathogenic crosstalk between tumor cells and pericytes. PLoS ONE 9, e101402 (2014).
Holash, J. et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science 284, 1994–1998 (1999).
Kaicker, S. et al. Thalidomide is anti-angiogenic in a xenograft model of neuroblastoma. Int. J. Oncol. 23, 1651–1655 (2003).
Kienast, Y. et al. Real-time imaging reveals the single steps of brain metastasis formation. Nat. Med. 16, 116–122 (2010).
Kim, E. S. et al. Potent VEGF blockade causes regression of coopted vessels in a model of neuroblastoma. Proc. Natl Acad. Sci. USA 99, 11399–11404 (2002).
Leenders, W. et al. Vascular endothelial growth factor-A determines detectability of experimental melanoma brain metastasis in GD-DTPA-enhanced MRI. Int. J. Cancer 105, 437–443 (2003).
Navis, A. C. et al. Effects of dual targeting of tumor cells and stroma in human glioblastoma xenografts with a tyrosine kinase inhibitor against c-MET and VEGFR2. PLoS ONE 8, e58262 (2013).
Vajkoczy, P. et al. Microtumor growth initiates angiogenic sprouting with simultaneous expression of VEGF, VEGF receptor-2, and angiopoietin-2. J. Clin. Invest. 109, 777–785 (2002).
Valiente, M. et al. Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell 156, 1002–1016 (2014).
Winkler, F. et al. Imaging glioma cell invasion in vivo reveals mechanisms of dissemination and peritumoral angiogenesis. Glia 57, 1306–1315 (2009).
Tufan, A. C. & Satiroglu-Tufan, N. L. The chick embryo chorioallantoic membrane as a model system for the study of tumor angiogenesis, invasion and development of anti-angiogenic agents. Curr. Cancer Drug Targets. 5, 249–266 (2005).
Gatter, K. & Brown, D. in Bone Marrow Diagnosis. An Illustrated Guide Ch. 2 (Wiley Blackwell, 2015).
Meadows, S. M. & Cleaver, O. Vascular patterning: coordinated signals keep blood vessels on track. Curr. Opin. Genet. Dev. 32, 86–91 (2015).
Rafii, S., Butler, J. M. & Ding, B. S. Angiocrine functions of organ-specific endothelial cells. Nature 529, 316–325 (2016).
Greenman, C. et al. Patterns of somatic mutation in human cancer genomes. Nature 446, 153–158 (2007).
Montana, V. & Sontheimer, H. Bradykinin promotes the chemotactic invasion of primary brain tumors. J. Neurosci. 31, 4858–4867 (2011).
Watkins, S. et al. Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells. Nat. Commun. 5, 4196 (2014).
Reymond, N. et al. Cdc42 promotes transendothelial migration of cancer cells through beta1 integrin. J. Cell Biol. 199, 653–668 (2012).
Lugassy, C., Eyden, B. P., Christensen, L. & Escande, J. P. Angio-tumoral complex in human malignant melanoma characterised by free laminin: ultrastructural and immunohistochemical observations. J. Submicrosc. Cytol. Pathol. 29, 19–28 (1997).
Winkler, F. Hostile takeover: how tumours hijack pre-existing vascular environments to thrive. J. Pathol. 242, 267–272 (2017).
Lugassy, C. et al. Angiotropism, pericytic mimicry and extravascular migratory metastasis in melanoma: an alternative to intravascular cancer dissemination. Cancer Microenviron. 7, 139–152 (2014).
Alexander, S., Weigelin, B., Winkler, F. & Friedl, P. Preclinical intravital microscopy of the tumour-stroma interface: invasion, metastasis, and therapy response. Curr. Opin. Cell Biol. 25, 659–671 (2013).
Jubb, A. M. et al. Vascular phenotypes in primary non-small cell lung carcinomas and matched brain metastases. Br. J. Cancer 104, 1877–1881 (2011).
Eefsen, R. L. et al. Histopathological growth pattern, proteolysis and angiogenesis in chemonaive patients resected for multiple colorectal liver metastases. J. Oncol. 2012, 907971 (2012).
Sakariassen, P. O. et al. Angiogenesis-independent tumor growth mediated by stem-like cancer cells. Proc. Natl Acad. Sci. USA 103, 16466–16471 (2006).
Ferguson, M. Angiogenesis in Human Lung Tumours. Thesis, Univ. Oxford (2008).
Drogat, B. et al. IRE1 signaling is essential for ischemia-induced vascular endothelial growth factor-A expression and contributes to angiogenesis and tumor growth in vivo. Cancer Res. 67, 6700–6707 (2007).
Adighibe, O. et al. Why some tumours trigger neovascularisation and others don't: the story thus far. Chin. J. Cancer 35, 18 (2016).
Hu, J. et al. Gene expression signature for angiogenic and nonangiogenic non-small-cell lung cancer. Oncogene 24, 1212–1219 (2005).
Goel, H. L. & Mercurio, A. M. VEGF targets the tumour cell. Nat. Rev. Cancer 13, 871–882 (2013).
Paez-Ribes, M. et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell 15, 220–231 (2009).
Lu, K. V. et al. VEGF inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complex. Cancer Cell 22, 21–35 (2012).
Sennino, B. et al. Suppression of tumor invasion and metastasis by concurrent inhibition of c-Met and VEGF signaling in pancreatic neuroendocrine tumors. Cancer Discov. 2, 270–287 (2012).
Depner, C. et al. EphrinB2 repression through ZEB2 mediates tumour invasion and anti-angiogenic resistance. Nat. Commun. 7, 12329 (2016).
Bovolenta, P., Rodriguez, J., & Esteve, P. Frizzled/RYK mediated signalling in axon guidance. Development 133, 4399–4408 (2006).
Lamouille, S., Xu, J. & Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 15, 178–196 (2014).
Coutts, A. S., Weston, L. & La Thangue, N. B. Actin nucleation by a transcription co-factor that links cytoskeletal events with the p53 response. Cell Cycle 9, 1511–1515 (2010).
Offersen, B. V., Pfeiffer, P., Hamilton-Dutoit, S. & Overgaard, J. Patterns of angiogenesis in non-small-cell lung carcinoma. Cancer 91, 1500–1509 (2001).
Pastorino, U. et al. Immunocytochemical markers in stage I lung cancer: relevance to prognosis. J. Clin. Oncol. 15, 2858–2865 (1997).
van Dam, P. J. et al. International consensus guidelines for scoring the histopathological growth patterns of liver metastasis. Br. J. Cancer 117, 1427–1441 (2017).
Bergers, G. & Hanahan, D. Modes of resistance to anti-angiogenic therapy. Nat. Rev. Cancer 8, 592–603 (2008).
Dey, N., De, P. & Brian, L. J. Evading anti-angiogenic therapy: resistance to anti-angiogenic therapy in solid tumors. Am. J. Transl Res. 7, 1675–1698 (2015).
Vasudev, N. S. & Reynolds, A. R. Anti-angiogenic therapy for cancer: current progress, unresolved questions and future directions. Angiogenesis 17, 471–494 (2014).
di Tomaso, T. E. et al. Glioblastoma recurrence after cediranib therapy in patients: lack of “rebound” revascularization as mode of escape. Cancer Res. 71, 19–28 (2011).
Franco, M., Paez-Ribes, M., Cortez, E., Casanovas, O. & Pietras, K. Use of a mouse model of pancreatic neuroendocrine tumors to find pericyte biomarkers of resistance to anti-angiogenic therapy. Horm. Metab. Res. 43, 884–889 (2011).
Helfrich, I. et al. Resistance to antiangiogenic therapy is directed by vascular phenotype, vessel stabilization, and maturation in malignant melanoma. J. Exp. Med. 207, 491–503 (2010).
Lee, C. H. & Motzer, R. J. Kidney cancer in 2016: The evolution of anti-angiogenic therapy for kidney cancer. Nat. Rev. Nephrol. 13, 69–70 (2017).
Guerin, E., Man, S., Xu, P. & Kerbel, R. S. A model of postsurgical advanced metastatic breast cancer more accurately replicates the clinical efficacy of antiangiogenic drugs. Cancer Res. 73, 2743–2748 (2013).
Szabo, V. et al. Mechanism of tumour vascularization in experimental lung metastases. J. Pathol. 235, 384–396 (2015).
Lee, E., Pandey, N. B. & Popel, A. S. Crosstalk between cancer cells and blood endothelial and lymphatic endothelial cells in tumour and organ microenvironment. Expert. Rev. Mol. Med. 17, e3 (2015).
Carmeliet, P. & Jain, R. K. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat. Rev. Drug Discov. 10, 417–427 (2011).
Seftor, R. E. et al. Tumor cell vasculogenic mimicry: from controversy to therapeutic promise. Am. J. Pathol. 181, 1115–1125 (2012).
Delgado-Bellido, D., Serrano-Saenz, S., Fernandez-Cortes, M. & Oliver, F. J. Vasculogenic mimicry signaling revisited: focus on non-vascular VE-cadherin. Mol. Cancer 16, 65 (2017).
Paulis, Y. W., Soetekouw, P. M., Verheul, H. M., Tjan-Heijnen, V. C. & Griffioen, A. W. Signalling pathways in vasculogenic mimicry. Biochim. Biophys. Acta 1806, 18–28 (2010).
Hendrix, M. J., Seftor, E. A., Hess, A. R. & Seftor, R. E. Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nat. Rev. Cancer 3, 411–421 (2003).
Sun, T. et al. Expression and functional significance of Twist1 in hepatocellular carcinoma: its role in vasculogenic mimicry. Hepatology 51, 545–556 (2010).
Liu, T. et al. HER2/neu expression correlates with vasculogenic mimicry in invasive breast carcinoma. J. Cell. Mol. Med. 17, 116–122 (2013).
Williamson, S. C. et al. Vasculogenic mimicry in small cell lung cancer. Nat. Commun. 7, 13322 (2016).
Maniotis, A. J. et al. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am. J. Pathol. 155, 739–752 (1999).
Acknowledgements
The authors thank their funding bodies, Cancer Research UK and Breast Cancer Research Foundation (A.L.H.); Norwegian Cancer Society and Northern Norway Health Region Authority (T.D.); Breast Cancer Now (A.R.R.); and Worldwide Cancer Research and the Canadian Breast Cancer Foundation (E.A.K.).
Author information
Authors and Affiliations
Contributions
T.D., F.P. and K.G. wrote the first drafts of the manuscript, while A.R.R., E.A.K., P.B.V., R.S.K. and A.L.H. provided comments and corrections before submission. All the authors contributed substantially to discussions of the content and to reviewing and/or editing the manuscript. With great sadness, Professor Kevin Gatter passed away during the preparation of the manuscript, which has been dedicated to his memory.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
RELATED LINKS
Supplementary information
Supplementary information
Supplementary information S1 (table) (DOC 500 kb)
Rights and permissions
About this article
Cite this article
Donnem, T., Reynolds, A., Kuczynski, E. et al. Non-angiogenic tumours and their influence on cancer biology. Nat Rev Cancer 18, 323–336 (2018). https://doi.org/10.1038/nrc.2018.14
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc.2018.14
This article is cited by
-
Antiangiogenic–immune-checkpoint inhibitor combinations: lessons from phase III clinical trials
Nature Reviews Clinical Oncology (2024)
-
Vascular mimicry as a facilitator of melanoma brain metastasis
Cellular and Molecular Life Sciences (2024)
-
Paranasal sinus angiosarcoma with facial paralysis as a novel manifestation: a case report and literature review
BMC Neurology (2023)
-
Crystal ribcage: a platform for probing real-time lung function at cellular resolution
Nature Methods (2023)
-
VE-Cadherin modulates β-catenin/TCF-4 to enhance Vasculogenic Mimicry
Cell Death & Disease (2023)