Key Points
-
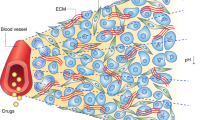
The transport of anticancer drugs throughout all regions of a solid tumour is a crucial factor in the effectiveness of the drug.
-
Generally, drugs are delivered by convection in the blood and then permeate vessel walls and penetrate the surrounding tissue by diffusion or convection.
-
The distribution of drug in tumour tissue is strongly influenced by the structure and function of the tumour vasculature, which typically has aberrant properties. The abnormal properties of the extracellular matrix in the tumour tissue can also limit drug distribution.
-
Physical properties of the drug, including size, charge, lipid solubility and surface properties (for nanoparticles), have major effects on the transport of the drug to tissue. The penetration distance of the drug into tissue also depends on solute binding and uptake rates in the tissue.
-
A number of methods have been developed for overcoming limitations in drug transport and thereby achieving improved therapeutic results.
-
In the design and usage of drugs, increased attention to transport properties has the potential to result in better treatments for solid tumours.
Abstract
The effectiveness of anticancer drugs in treating a solid tumour is dependent on delivery of the drug to virtually all cancer cells in the tumour. The distribution of drug in tumour tissue depends on the plasma pharmacokinetics, the structure and function of the tumour vasculature and the transport properties of the drug as it moves through microvessel walls and in the extravascular tissue. The aim of this Review is to provide a broad, balanced perspective on the current understanding of drug transport to tumour cells and on the progress in developing methods to enhance drug delivery. First, the fundamental processes of solute transport in blood and tissue by convection and diffusion are reviewed, including the dependence of penetration distance from vessels into tissue on solute binding or uptake in tissue. The effects of the abnormal characteristics of tumour vasculature and extravascular tissue on these transport properties are then discussed. Finally, methods for overcoming limitations in drug transport and thereby achieving improved therapeutic results are surveyed.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bast, R. C. et al. Holland-Frei Cancer Medicine 9th edn (Wiley-Blackwell, 2017).
Swabb, E. A., Wei, J. & Gullino, P. M. Diffusion and convection in normal and neoplastic tissues. Cancer Res. 34, 2814–2822 (1974). In this early account of how tissue glycosaminoglycan content can affect solute transport by convection compared with by diffusion in tumours, the authors suggest that degradation of glycosaminoglycan with hyaluronidase could improve solute transport in tumours.
Jain, R. K. Transport of molecules across tumor vasculature. Cancer Metastasis Rev. 6, 559–593 (1987). This comprehensive review discusses the transport properties of microvessel walls with respect to their importance for drugs of various sizes.
Jain, R. K. Transport of molecules in the tumor interstitium: a review. Cancer Res. 47, 3039–3051 (1987). This review focuses on factors that impede solute transport in the interstitium of tumours and shows how high IFP and lack of functioning lymphatics contribute to reduced transport.
Jain, R. K. The Eugene M. Landis Award Lecture 1996. Delivery of molecular and cellular medicine to solid tumors. Microcirculation 4, 1–23 (1997).
Chauhan, V. P., Stylianopoulos, T., Boucher, Y. & Jain, R. K. Delivery of molecular and nanoscale medicine to tumors: transport barriers and strategies. Annu. Rev. Chem. Biomol. Eng. 2, 281–298 (2011).
Chauhan, V. P. & Jain, R. K. Strategies for advancing cancer nanomedicine. Nat. Mater. 12, 958–962 (2013).
El-Kareh, A. W. & Secomb, T. W. Theoretical models for drug delivery to solid tumors. Crit. Rev. Biomed. Eng. 25, 503–571 (1997).
Ribatti, D., Nico, B., Crivellato, E. & Vacca, A. The structure of the vascular network of tumors. Cancer Lett. 248, 18–23 (2007).
Maeda, H., Wu, J., Sawa, T., Matsumura, Y. & Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J. Control. Release 65, 271–284 (2000). This review discusses the features that enhance large drug and nanoparticle transport to tumours (the EPR effect) and the roles of VEGF, nitric oxide and other vasoactive and pro-angiogenic factors in these processes.
Minchinton, A. I. & Tannock, I. F. Drug penetration in solid tumours. Nat. Rev. Cancer 6, 583–592 (2006). This Review examines the features of the tumour microenvironment that inhibit small molecule transport and illustrates that the barriers are substantial, even for small drugs.
Chary, S. R. & Jain, R. K. Direct measurement of interstitial convection and diffusion of albumin in normal and neoplastic tissues by fluorescence photobleaching. Proc. Natl Acad. Sci. USA 86, 5385–5389 (1989).
Baxter, L. T. & Jain, R. K. Transport of fluid and macromolecules in tumors. I. Role of interstitial pressure and convection. Microvasc. Res. 37, 77–104 (1989).
Levick, J. R. An Introduction to Cardiovascular Physiology 4th edn (Hodder Arnold, 2003).
Pardridge, W. M. The blood-brain barrier: bottleneck in brain drug development. NeuroRx 2, 3–14 (2005).
Primeau, A. J., Rendon, A., Hedley, D., Lilge, L. & Tannock, I. F. The distribution of the anticancer drug doxorubicin in relation to blood vessels in solid tumors. Clin. Cancer Res. 11, 8782–8788 (2005).
Dewhirst, M. W., Cao, Y. & Moeller, B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat. Rev. Cancer 8, 425–437 (2008).
Wilson, W. R. & Hay, M. P. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer 11, 393–410 (2011).
Thomlinson, R. H. & Gray, L. H. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br. J. Cancer 9, 539–549 (1955).
Vaupel, P. & Mayer, A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 26, 225–239 (2007).
Brown, J. M. & Giaccia, A. J. Tumour hypoxia: the picture has changed in the 1990s. Int. J. Radiat. Biol. 65, 95–102 (1994).
Hicks, K. O. et al. Use of three-dimensional tissue cultures to model extravascular transport and predict in vivo activity of hypoxia-targeted anticancer drugs. J. Natl Cancer Inst. 98, 1118–1128 (2006).
Baish, J. W. et al. Scaling rules for diffusive drug delivery in tumor and normal tissues. Proc. Natl Acad. Sci. USA 108, 1799–1803 (2011).
El-Kareh, A. W. & Secomb, T. W. A theoretical model for intraperitoneal delivery of cisplatin and the effect of hyperthermia on drug penetration distance. Neoplasia 6, 117–127 (2004).
Senger, D. R. et al. Tumor-cells secrete a vascular-permeability factor that promotes accumulation of ascites-fluid. Science 219, 983–985 (1983).
Dewhirst, M. W. & Ashcraft, K. A. Implications of increase in vascular permeability in tumors by VEGF: a commentary on the pioneering work of Harold Dvorak. Cancer Res. 76, 3118–3121 (2016).
Sevick, E. M. & Jain, R. K. Geometric resistance to blood flow in solid tumors perfused ex vivo — effects of tumor size and perfusion pressure. Cancer Res. 49, 3506–3512 (1989).
Sevick, E. M. & Jain, R. K. Viscous resistance to blood flow in solid tumors — effect of hematocrit on intratumor blood viscosity. Cancer Res. 49, 3513–3519 (1989).
Dewhirst, M. W. et al. Effects of the calcium-channel blocker flunarizine on the hemodynamics and oxygenation of tumor microvasculature. Radiat. Res. 132, 61–68 (1992).
Kavanagh, B. D., Coffey, B. E., Needham, D., Hochmuth, R. M. & Dewhirst, M. W. The effect of flunarizine on erythrocyte suspension viscosity under conditions of extreme hypoxia, low pH, and lactate treatment. Br. J. Cancer 67, 734–741 (1993).
Falk, P. Patterns of vasculature in 2 pairs of related fibrosarcomas in rat and their relation to tumor responses to single large doses of radiation. Eur. J. Cancer 14, 237–250 (1978).
Dewhirst, M. W. et al. Quantification of longitudinal tissue pO2 gradients in window chamber tumours: impact on tumour hypoxia. Br. J. Cancer 79, 1717–1722 (1999).
Less, J. R., Skalak, T. C., Sevick, E. M. & Jain, R. K. Microvascular architecture in a mammary-carcinoma — branching patterns and vessel dimensions. Cancer Res. 51, 265–273 (1991).
Mayrovitz, H. N. & Roy, J. Microvascular blood flow: evidence indicating a cubic dependence on arteriolar diameter. Am. J. Physiol. 245, H1031–H1038 (1983).
Dewhirst, M. W. et al. Morphologic and hemodynamic comparison of tumor and healing normal tissue microvasculature. Int. J. Radiat. Oncol. Biol. Phys. 17, 91–99 (1989).
Secomb, T. W., Hsu, R., Dewhirst, M. W., Klitzman, B. & Gross, J. F. Analysis of oxygen transport to tumor tissue by microvascular networks. Int. J. Radiat. Oncol. Biol. Phys. 25, 481–489 (1993). This study uses a mathematical model to show that the irregular structure of tumour microcirculation leads to a wide distribution of tissue oxygen levels, implying that heterogeneous responses to radiation and some types of chemotherapy can be dependent on hypoxia.
Jain, R. K. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307, 58–62 (2005). This commentary proposes a novel rationale for the use of antiangiogenic drugs; namely, that enhanced drug delivery could occur because of pruning of nonfunctional microvasculature coupled with a reduction in the diameter of remaining vasculature.
Winkler, F. et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell 6, 553–563 (2004).
Dewhirst, M. W. et al. Perivascular oxygen tensions in a transplantable mammary tumor growing in a dorsal flap window chamber. Radiat. Res. 130, 171–182 (1992).
Helmlinger, G., Yuan, F., Dellian, M. & Jain, R. K. Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nat. Med. 3, 177–182 (1997).
Kimura, H. et al. Fluctuations in red cell flux in tumor microvessels can lead to transient hypoxia and reoxygenation in tumor parenchyma. Cancer Res. 56, 5522–5528 (1996).
Dewhirst, M. W. Relationships between cycling hypoxia, HIF-1, angiogenesis and oxidative stress. Radiat. Res. 172, 653–665 (2009).
Sorg, B. S., Hardee, M. E., Agarwal, N., Moeller, B. J. & Dewhirst, M. W. Spectral imaging facilitates visualization and measurements of unstable and abnormal microvascular oxygen transport in tumors. J. Biomed. Opt. 13, 014026 (2008).
Yeh, C. H. et al. Optical-resolution photoacoustic microscopy of the metabolic rate of oxygen in a mouse renal tumor model. Proc. SPIE 9323, 93233H (2015).
Feindel, W. & Perot, P. Red cerebral veins — a report on arteriovenous shunts in tumors and cerebral scars. J. Neurosurg. 22, 315–325 (1965).
Forman, W. H., Green, J. D. & Oberheu, V. Arteriovenous shunting in renal pelvic tumors. South. Med. J. 68, 992–993 (1975).
Numata, K. et al. Flow characteristics of hepatic-tumors at color Doppler sonography — correlation with arteriographic findings. Am. J. Roentgenol. 160, 515–521 (1993).
Pries, A. R., Hopfner, M., le Noble, F., Dewhirst, M. W. & Secomb, T. W. The shunt problem: control of functional shunting in normal and tumour vasculature. Nat. Rev. Cancer 10, 587–593 (2010). This Opinion article proposes that signal propagation along walls of blood vessels is impaired in tumours, resulting in dysfunctional microcirculation, and that antiangiogenic treatment may restore vascular communication and thereby normalize vascular function.
Pries, A. R., Secomb, T. W. & Gaehtgens, P. Structural adaptation and stability of microvascular networks: theory and simulations. Am. J. Physiol. 275, H349–H360 (1998).
Figueroa, X. F. & Duling, B. R. Gap junctions in the control of vascular function. Antioxid. Redox Signal. 11, 251–266 (2009).
Yuan, F. et al. Vascular-permeability in a human tumor xenograft - molecular-size dependence and cutoff size. Cancer Res. 55, 3752–3756 (1995).
Yuan, F. et al. Microvascular permeability and interstitial penetration of sterically stabilized (stealth) liposomes in a human tumor xenograft. Cancer Res. 54, 3352–3356 (1994). This first report of permeability coefficient measurements for nanoparticles (sterically stabilized liposomes) in tumour tissue compared with normal tissue shows that extravasated liposomes remain in the perivascular space for many days.
Yokoi, K. et al. Capillary-wall collagen as a biophysical marker of nanotherapeutic permeability into the tumor microenvironment. Cancer Res. 74, 4239–4246 (2014).
Manzoor, A. A. et al. Overcoming limitations in nanoparticle drug delivery: triggered, intravascular release to improve drug penetration into tumors. Cancer Res. 72, 5566–5575 (2012). This report outlines a strategy to increase drug transport to tumours more efficiently than through the EPR effect by use of hyperthermia to achieve thermosensitive liposomal drug release within the tumour vasculature.
Miller, M. A. et al. Predicting therapeutic nanomedicine efficacy using a companion magnetic resonance imaging nanoparticle. Sci. Transl Med. 7, 314ra183 (2015).
Bertrand, N., Wu, J., Xu, X., Kamaly, N. & Farokhzad, O. C. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 66, 2–25 (2014).
Lee, H. et al. 64Cu-MM-302 positron emission tomography quantifies variability of enhanced permeability and retention of nanoparticles in relation to treatment response in patients with metastatic breast cancer. Clin. Cancer Res. 23, 4190–4202 (2017).
Prabhakar, U. et al. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 73, 2412–2417 (2013).
Sevick, E. M. & Jain, R. K. Measurement of capillary filtration coefficient in a solid tumor. Cancer Res. 51, 1352–1355 (1991).
Chang, Q. et al. Biodistribution of cisplatin revealed by imaging mass cytometry identifies extensive collagen binding in tumor and normal tissues. Sci. Rep. 6, 36641 (2016).
Krol, A., Maresca, J., Dewhirst, M. W. & Yuan, F. Available volume fraction of macromolecules in the extravascular space of a fibrosarcoma: implications for drug delivery. Cancer Res. 59, 4136–4141 (1999).
Yuan, F., Krol, A. & Tong, S. Available space and extracellular transport of macromolecules: effects of pore size and connectedness. Ann. Biomed. Engineer. 29, 1150–1158 (2001). This study shows that collapse of tumour microvessels is not the result of high IFP, but that the hyaluronic acid component of tumour ECM plays a role in compressing microvessels.
Provenzano, P. P. et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 21, 418–429 (2012).
Stylianopoulos, T. et al. Causes, consequences, and remedies for growth-induced solid stress in murine and human tumors. Proc. Natl Acad. Sci. USA 109, 15101–15108 (2012).
Chauhan, V. P. et al. Compression of pancreatic tumor blood vessels by hyaluronan is caused by solid stress and not interstitial fluid pressure. Cancer Cell 26, 14–15 (2014).
Levin, V. A. Relationship of octanol/water partition coefficient and molecular weight to rat brain capillary permeability. J. Med. Chem. 23, 682–684 (1980).
Pruijn, F. B., Patel, K., Hay, M. P., Wilson, W. R. & Hicks, K. O. Prediction of tumour tissue diffusion coefficients of hypoxia-activated prodrugs from physicochemical parameters. Aust. J. Chem. 61, 687–693 (2008).
Norvaisas, P. & Ziemys, A. The role of payload hydrophobicity in nanotherapeutic pharmacokinetics. J. Pharm. Sci. 103, 2147–2156 (2014).
Wu, N. Z. et al. Increased microvascular permeability contributes to preferential accumulation of Stealth liposomes in tumor tissue. Cancer Res. 53, 3765–3770 (1993).
Dreher, M. R. et al. Tumor vascular permeability, accumulation, and penetration of macromolecular drug carriers. J. Natl Cancer Inst. 98, 335–344 (2006). This is the first paper to systematically evaluate the size dependence of macromolecular transport across tumour microvasculature and into the interstitial space.
Kyle, A. H., Huxham, L. A., Chiam, A. S., Sim, D. H. & Minchinton, A. I. Direct assessment of drug penetration into tissue using a novel application of three-dimensional cell culture. Cancer Res. 64, 6304–6309 (2004).
Stylianopoulos, T. & Jain, R. K. Design considerations for nanotherapeutics in oncology. Nanomedicine 11, 1893–1907 (2015).
Folkman, J. Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 285, 1182–1186 (1971).
Hurwitz, H. et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 350, 2335–2342 (2004).
Yuan, F. et al. Time-dependent vascular regression and permeability changes in established human tumor xenografts induced by an anti-vascular endothelial growth factor vascular permeability factor antibody. Proc. Natl Acad. Sci. USA 93, 14765–14770 (1996).
Jain, R. K. & Booth, M. F. What brings pericytes to tumor vessels? J. Clin. Invest. 112, 1134–1136 (2003).
Abramsson, A., Lindblom, P. & Betsholtz, C. Endothelial and nonendothelial sources of PDGF-B regulate pericyte recruitment and influence vascular pattern formation in tumors. J. Clin. Invest. 112, 1142–1151 (2003).
Hellstrom, M., Kalen, M., Lindahl, P., Abramsson, A. & Betsholtz, C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 126, 3047–3055 (1999).
Lin, P. N. et al. Inhibition of tumor angiogenesis using a soluble receptor establishes a role for Tie2 in pathologic vascular growth. J. Clin. Invest. 100, 2072–2078 (1997).
Wong, A. L. et al. Tie2 expression and phosphorylation in angiogenic and quiescent adult tissues. Circul. Res. 81, 567–574 (1997).
Goel, S. et al. Effects of vascular-endothelial protein tyrosine phosphatase inhibition on breast cancer vasculature and metastatic progression. J. Natl Cancer Inst. 105, 1188–1201 (2013).
Willett, C. G. et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat. Med. 10, 145–147 (2004). This study demonstrates potent antiangiogenic effects of bevacizumab in human patients with rectal cancer and suggests that the tumour vasculature became more efficient after treatment.
Tong, R. T. et al. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 64, 3731–3736 (2004).
Wong, A. L. A. et al. Phase Ib/II randomized, open-label study of doxorubicin and cyclophosphamide with or without low-dose, short-course sunitinib in the pre-operative treatment of breast cancer. Oncotarget 7, 64089–64099 (2016).
Li, W. et al. Gold nanoparticle-mediated targeted delivery of recombinant human endostatin normalizes tumour vasculature and improves cancer therapy. Sci. Rep. 6, 11 (2016).
Luan, X. et al. Tumor priming using metronomic chemotherapy with neovasculature-targeted, nanoparticulate paclitaxel. Biomaterials 95, 60–73 (2016).
Dickson, P. V. et al. Revacizumab-induced transient remodeling of the vasculature in neuroblastoma xenografts results in improved delivery and efficacy of systemically administered chemotherapy. Clin. Cancer Res. 13, 3942–3950 (2007).
Liu, J. Q. et al. TGF-β blockade improves the distribution and efficacy of therapeutics in breast carcinoma by normalizing the tumor stroma. Proc. Natl Acad. Sci. USA 109, 16618–16623 (2012).
Arjaans, M. et al. Bevacizumab-induced normalization of blood vessels in tumors hampers antibody uptake. Cancer Res. 73, 3347–3355 (2013).
Doi, Y. et al. Improvement of intratumor microdistribution of PEGylated liposome via tumor priming by metronomic S-1 dosing. Int. J. Nanomed. 11, 5573–5582 (2016).
Sorensen, A. G. et al. Increased survival of glioblastoma patients who respond to antiangiogenic therapy with elevated blood perfusion. Cancer Res. 72, 402–407 (2012).
Jain, R. K. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J. Clin. Oncol. 31, 2205–2218 (2013).
Jain, R. K. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell 26, 605–622 (2014).
Tailor, T. D. et al. Effect of pazopanib on tumor microenvironment and liposome delivery. Mol. Cancer Ther. 9, 1798–1808 (2010).
Vlahovic, G., Rabbani, Z. N., Herndon, J. E., Dewhirst, M. W. & Vujaskovic, Z. Treatment with Imatinib in NSCLC is associated with decrease of phosphorylated PDGFR-β and VEGF expression, decrease in interstitial fluid pressure and improvement of oxygenation. Br. J. Cancer 95, 1013–1019 (2006).
Chauhan, V. P. et al. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat. Nanotechnol. 7, 383–388 (2012).
Betof, A. S. et al. Modulation of murine breast tumor vascularity, hypoxia, and chemotherapeutic response by exercise. J. Natl Cancer Inst. 107, djv040 (2015).
Jones, L. W. et al. Exercise modulation of the host-tumor interaction in an orthotopic model of murine prostate cancer. J. Appl. Physiol. 113, 263–272 (2012).
Jones, L. W. et al. Effect of aerobic exercise on tumor physiology in an animal model of human breast cancer. J. Appl. Physiol. 108, 343–348 (2010).
Schadler, K. L. et al. Tumor vessel normalization after aerobic exercise enhances chemotherapeutic efficacy. Oncotarget 7, 65429–65440 (2016).
Greenaway, J. et al. Thrombospondin-1 inhibits VEGF levels in the ovary directly by binding and internalization via the low density lipoprotein receptor-related protein-1 (LRP-1). J. Cell. Physiol. 210, 807–818 (2007).
Edwards, A. K., Olariu, I., Nakamura, D. S., Ahn, S. H. & Tayade, C. Chronic effects of an anti-angiogenic thrombospondin-1 mimetic peptide, ABT-898, on female mouse reproductive outcomes. Reprod. Biol. Endocrinol. 14, 10 (2016).
Griffon-Etienne, G., Boucher, Y., Brekken, C., Suit, H. D. & Jain, R. K. Taxane-induced apoptosis decompresses blood vessels and lowers interstitial fluid pressure in solid tumors: clinical implications. Cancer Res. 59, 3776–3782 (1999).
Geretti, E. et al. Cyclophosphamide-mediated tumor priming for enhanced delivery and antitumor activity of HER2-targeted liposomal doxorubicin (MM-302). Mol. Cancer Ther. 14, 2060–2071 (2015).
Jang, S. H., Wientjes, M. G. & Au, J. L. S. Enhancement of paclitaxel delivery to solid tumors by apoptosis-inducing pretreatment: effect of treatment schedule. J. Pharmacol. Exp. Ther. 296, 1035–1042 (2001).
Lu, D., Wientjes, M. G., Lu, Z. & Au, J. L. S. Tumor priming enhances delivery and efficacy of nanomedicines. J. Pharmacol. Exp. Ther. 322, 80–88 (2007).
Jang, J. K. et al. Cytoreductive chemotherapy improves the biodistribution of antibodies directed against tumor necrosis in murine solid tumor models. Mol. Cancer Ther. 12, 2827–2836 (2013).
Hylander, B. L. et al. Tumor priming by Apo2L/TRAIL reduces interstitial fluid pressure and enhances efficacy of liposomal gemcitabine in a patient derived xenograft tumor model. J. Control. Release 217, 160–169 (2015).
Edgington, L. E. et al. Noninvasive optical imaging of apoptosis by caspase-targeted activity-based probes. Nat. Med. 15, 967–973 (2009).
Lee, K. C. et al. Prospective early response imaging biomarker for neoadjuvant breast cancer chemotherapy. Clin. Cancer Res. 13, 443–450 (2007).
Szakacs, G., Paterson, J. K., Ludwig, J. A., Booth-Genthe, C. & Gottesman, M. M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 5, 219–234 (2006).
Xu, R. et al. An injectable nanoparticle generator enhances delivery of cancer therapeutics. Nat. Biotechnol. 34, 414–418 (2016).
Geisow, M. J., D'Arcy Hart, P. & Young, M. R. Temporal changes of lysosome and phagosome pH during phagolysosome formation in macrophages: studies by fluorescence spectroscopy. J. Cell Biol. 89, 645–652 (1981).
Thiebaut, F. et al. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc. Natl Acad. Sci. USA 84, 7735–7738 (1987).
MacKay, J. A. et al. Self-assembling chimeric polypeptide-doxorubicin conjugate nanoparticles that abolish tumours after a single injection. Nat. Mater. 8, 993–999 (2009).
Jacobetz, M. A. et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut 62, 112–120 (2013).
Thompson, C. B. et al. Enzymatic depletion of tumor hyaluronan induces antitumor responses in preclinical animal models. Mol. Cancer Ther. 9, 3052–3064 (2010).
Singha, N. C. et al. Tumor-associated hyaluronan limits efficacy of monoclonal antibody therapy. Mol. Cancer Ther. 14, 523–532 (2015).
Hingorani, S. R. et al. Phase Ib study of PEGylated recombinant human hyaluronidase and gemcitabine in patients with advanced pancreatic cancer. Clin. Cancer Res. 22, 2848–2854 (2016). This is the first clinical evaluation of the use of hyaluronidase to enhance drug delivery to pancreatic cancer.
Olive, K. P. et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324, 1457–1461 (2009).
Pietras, K. et al. Inhibition of platelet-derived growth factor receptors reduces interstitial hypertension and increases transcapillary transport in tumors. Cancer Res. 61, 2929–2934 (2001).
Vlahovic, G. et al. Treatment with imatinib improves drug delivery and efficacy in NSCLC xenografts. Br. J. Cancer 97, 735–740 (2007).
Lee, S. J. & Wang, J. Y. J. Exploiting the promiscuity of imatinib BMC Biol. 8, 30 (2010).
Pardali, E. & ten Dijke, P. Transforming growth factor-β signaling and tumor angiogenesis. Front. Biosci. 14, 4848–4861 (2009).
Diop-Frimpong, B., Chauhan, V. P., Krane, S., Boucher, Y. & Jain, R. K. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc. Natl Acad. Sci. USA 108, 2909–2914 (2011).
Bago, J. R. et al. Therapeutically engineered induced neural stem cells are tumour-homing and inhibit progression of glioblastoma. Nat. Commun. 7, 10593 (2016).
Miller, M. A. et al. Tumour-associated macrophages act as a slow-release reservoir of nano-therapeutic Pt(IV) pro-drug. Nat. Commun. 6, 8692 (2015).
Dewhirst, M., Stauffer, P., Das, S., Craciunescu, O. & Vujaskovic, Z. in Clinical Radiation Oncology (eds Gunderson, L. & Tepper, J.) 381–398 (Elsevier, 2016).
Kong, G., Braun, R. D. & Dewhirst, M. W. Hyperthermia enables tumor-specific nanoparticle delivery: effect of particle size. Cancer Res. 60, 4440–4445 (2000). This study demonstrates that hyperthermia increases pore sizes in tumour microvessels, which yields enhanced nanoparticle delivery.
Cope, D. A., Dewhirst, M. W., Friedman, H. S., Bigner, D. D. & Zalutsky, M. R. Enhanced delivery of a monoclonal-antibody F(ab')2 fragment to subcutaneous human glioma xenografts using local hyperthermia. Cancer Res. 50, 1803–1809 (1990).
Schuster, J. M. et al. Hyperthermic modulation of radiolabeled antibody uptake in a human glioma xenograft and normal tissues. Int. J. Hyperthermia 11, 59–72 (1995).
Krol, A., Dewhirst, M. W. & Yuan, F. Effects of cell damage and glycosaminoglycan degradation on available extravascular space of different dextrans in a rat fibrosarcoma. Int. J. Hyperthermia 19, 154–164 (2003).
Dudar, T. E. & Jain, R. K. Differential response of normal and tumor microcirculation to hyperthermia. Cancer Res. 44, 605–612 (1984).
Song, C. W. Effect of local hyperthermia on blood flow and microenvironment — a review. Cancer Res. 44, 4721–4730 (1984).
Song, C. W., Rhee, J. G. & Levitt, S. H. Blood flow in normal tissues and tumors during hyperthermia. J. Natl Cancer Inst. 64, 119–124 (1980).
Meyer, R. E., Braun, R. D., Rosner, G. L. & Dewhirst, M. W. Local 42 degrees C hyperthermia improves vascular conductance of the R3230Ac rat mammary adenocarcinoma during sodium nitroprusside infusion. Radiat. Res. 154, 196–201 (2000).
Vujaskovic, Z. et al. Temperature-dependent changes in physiologic parameters of spontaneous canine soft tissue sarcomas after combined radiotherapy and hyperthermia treatment. Int. J. Radiat. Oncol. Biol. Phys. 46, 179–185 (2000).
Kong, G. & Dewhirst, M. W. Hyperthermia and liposomes. Int. J. Hyperthermia 15, 345–370 (1999).
Kong, G. et al. Efficacy of liposomes and hyperthermia in a human tumor xenograft model: Importance of triggered drug release. Cancer Res. 60, 6950–6957 (2000).
Matteucci, M. L. et al. Hyperthermia increases accumulation of technetium-99m-labeled liposomes in feline sarcomas. Clin. Cancer Res. 6, 3748–3755 (2000).
McDaniel, J. R., Dewhirst, M. W. & Chilkoti, A. Actively targeting solid tumours with thermoresponsive drug delivery systems that respond to mild hyperthermia. Int. J. Hyperthermia 29, 501–510 (2013).
McDaniel, J. R. et al. Rational design of “heat seeking” drug loaded polypeptide nanoparticles that thermally target solid tumors. Nano Lett. 14, 2890–2895 (2014).
Winslow, T. B. et al. A pilot study of the effects of mild systemic heating on human head and neck tumour xenografts: analysis of tumour perfusion, interstitial fluid pressure, hypoxia and efficacy of radiation therapy. Int. J. Hyperthermia 31, 693–701 (2015).
Xu, Y. et al. Fever-range whole body hyperthermia increases the number of perfused tumor blood vessels and therapeutic efficacy of liposomally encapsulated doxorubicin. Int. J. Hyperthermia 23, 513–527 (2007).
Yatvin, M. B., Weinstein, J. N., Dennis, W. H. & Blumenthal, R. Design of liposomes for enhanced local release of drugs by hyperthermia. Science 202, 1290–1293 (1978). This study is the first to suggest that a thermal trigger could enhance drug delivery from liposomes constructed of lipids that undergo a temperature-dependent phase transition.
Gaber, M. H. et al. Thermosensitive liposomes: extravasation and release of contents in tumor microvascular networks. Int. J. Radiat. Oncol. Biol. Phys. 36, 1177–1187 (1996).
Yarmolenko, P. S. et al. Comparative effects of thermosensitive doxorubicin-containing liposomes and hyperthermia in human and murine tumours. Int. J. Hyperthermia 26, 485–498 (2010).
Lokerse, W. J. M. et al. In depth study on thermosensitive liposomes: optimizing formulations for tumor specific therapy and in vitro to in vivo relations. Biomaterials 82, 138–150 (2016).
Lu, T., Lokerse, W. J. M., Seynhaeve, A. L. B., Koning, G. A. & ten Hagen, T. L. M. Formulation and optimization of idarubicin thermosensitive liposomes provides ultrafast triggered release at mild hyperthermia and improves tumor response. J. Control. Release 220, 425–437 (2015).
Lindner, L. H. et al. Dual role of hexadecylphosphocholine (miltefosine) in thermosensitive liposomes: active ingredient and mediator of drug release. J. Control. Release 125, 112–120 (2008).
Needham, D., Anyarambhatla, G., Kong, G. & Dewhirst, M. W. A new temperature-sensitive liposome for use with mild hyperthermia: characterization and testing in a human tumor xenograft model. Cancer Res. 60, 1197–1201 (2000).
Zagar, T. M. et al. Two phase I dose-escalation/pharmacokinetics studies of low temperature liposomal doxorubicin (LTLD) and mild local hyperthermia in heavily pretreated patients with local regionally recurrent breast cancer. Int. J. Hyperthermia 30, 285–294 (2014).
Poon, R. T. P. & Borys, N. Lyso-thermosensitive liposomal doxorubicin: an adjuvant to increase the cure rate of radiofrequency ablation in liver cancer. Future Oncol. 7, 937–945 (2011).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02112656 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02181075 (2017).
Dou, Y. N. et al. Heat-activated thermosensitive liposomal cisplatin (HTLC) results in effective growth delay of cervical carcinoma in mice. J. Control. Release 178, 69–78 (2014).
Kakinuma, K. et al. Drug delivery to the brain using thermosensitive liposome and local hyperthermia. Int. J. Hyperthermia 12, 157–165 (1996).
Weinstein, J. N., Magin, R. L., Yatvin, M. B. & Zaharko, D. S. Liposomes and local hyperthermia — selective delivery of methotrexate to heated tumors. Science 204, 188–191 (1979).
Chelvi, T. P., Jain, S. K. & Ralhan, R. Hyperthermia-mediated targeted delivery of thermosensitive liposome-encapsulated melphalan in murine tumors. Oncol. Res. 7, 393–398 (1995).
Ponce, A. M. et al. Magnetic resonance imaging of temperature-sensitive liposome release: drug dose painting and antitumor effects. J. Natl Cancer Inst. 99, 53–63 (2007).
Paoli, E. E. et al. An optical and microPET assessment of thermally-sensitive liposome biodistribution in the Met-1 tumor model: importance of formulation. J. Control. Release 143, 13–22 (2010).
Viglianti, B. L. et al. In vivo monitoring of tissue pharmacokinetics of liposome/drug using MRI: illustration of targeted delivery. Magn. Reson. Med. 51, 1153–1162 (2004).
Grull, H. & Langereis, S. Hyperthermia-triggered drug delivery from temperature-sensitive liposomes using MRI-guided high intensity focused ultrasound. J. Control. Release 161, 317–327 (2012).
Peller, M. et al. Surrogate MRI markers for hyperthermia-induced release of doxorubicin from thermosensitive liposomes in tumors. J. Control. Release 237, 138–146 (2016).
Ranjan, A. et al. Image-guided drug delivery with magnetic resonance guided high intensity focused ultrasound and temperature sensitive liposomes in a rabbit Vx2 tumor model. J. Control. Release 158, 487–494 (2012).
Perez, H. L. et al. Antibody-drug conjugates: current status and future directions. Drug Discov. Today 19, 869–881 (2014).
Agarwal, P. & Bertozzi, C. R. Site-specific antibody-drug conjugates: the nexus of bioorthogonal chemistry, protein engineering, and drug development. Bioconjugate Chem. 26, 176–192 (2015).
Vasalou, C., Helmlinger, G. & Gomes, B. A mechanistic tumor penetration model to guide antibody drug conjugate design. PLoS ONE 10, e0118977 (2015).
Erickson, H. K. et al. Antibody-maytansinoid conjugates are activated in targeted cancer cells by lysosomal degradation and linker-dependent intracellular processing. Cancer Res. 66, 4426–4433 (2006).
Maass, K. F., Kulkarni, C., Betts, A. M. & Wittrup, K. D. Determination of cellular processing rates for a trastuzumab-maytansinoid antibody-drug conjugate (ADC) highlights key parameters for ADC design. AAPS J. 18, 635–646 (2016).
Diamantis, N. & Banerji, U. Antibody-drug conjugates — an emerging class of cancer treatment. Br. J. Cancer 114, 362–367 (2016).
Rich, L. J. et al. Enhanced tumour perfusion following treatment with water-filtered IR-A radiation to the thorax in a patient with head and neck cancer. Int. J. Hyperthermia 32, 539–542 (2016).
Aziz, S. A. et al. Vascularity of primary and metastatic renal cell carcinoma specimens. J. Transl Med. 11, 15 (2013).
Liu, J. Q. et al. PDGF-D improves drug delivery and efficacy via vascular normalization, but promotes lymphatic metastasis by activating CXCR4 in breast cancer. Clin. Cancer Res. 17, 3638–3648 (2011).
Shen, G. et al. SKLB1002, a novel inhibitor of VEGF receptor 2 signaling, induces vascular normalization to improve systemically administered chemotherapy efficacy. Neoplasma 59, 486–493 (2012).
Viglianti, B. L. et al. Systemic anti-tumour effects of local thermally sensitive liposome therapy. Int. J. Hyperthermia 30, 385–392 (2014).
Wu, J., Gong, G. H., Cui, Y. & Li, R. J. Intratumor partitioning and texture analysis of dynamic contrast-enhanced (DCE)-MRI identifies relevant tumor subregions to predict pathological response of breast cancer to neoadjuvant chemotherapy. J. Magn. Reson. Imag. 76, 1107–1115 (2016).
Pascal, J. et al. Mechanistic patient-specific predictive correlation of tumor drug response with microenvironment and perfusion measurements. Proc. Natl Acad. Sci. USA 110, 14266–14271 (2013).
Koay, E. J. et al. Transport properties of pancreatic cancer describe gemcitabine delivery and response. J. Clin. Invest. 124, 1525–1536 (2014).
Wientjes, M. G., Yeung, B. Z., Lu, Z., Wientjes, M. G. & Au, J. L. Predicting diffusive transport of cationic liposomes in 3-dimensional tumor spheroids. J. Control. Release 192, 10–18 (2014).
Kerr, D. J., Kerr, A. M., Freshney, R. I. & Kaye, S. B. Comparative intracellular uptake of adriamycin and 4′-deoxydoxorubicin by non-small cell lung tumor cells in culture and its relationship to cell survival. Biochem. Pharmacol. 35, 2817–2823 (1986).
Dewhirst, M. W., Secomb, T. W., Ong, E. T., Hsu, R. & Gross, J. F. Determination of local oxygen consumption rates in tumors. Cancer Res. 54, 3333–3336 (1994).
Acknowledgements
This work was supported by National Institutes of Health (NIH) grants CA040355 and HL034555.
Author information
Authors and Affiliations
Contributions
M.W.D. accepted the commission to prepare this Review and prepared the outline in collaboration with T.W.S. M.W.D. researched data in collaboration with T.W.S. M.W.D and T.W.S. contributed equally to writing and revising the text.
Corresponding author
Ethics declarations
Competing interests
T.W.S. has no conflicts of interest. M.W.D. was involved in the development of the thermally sensitive liposome described in this paper and has stock in Celsion Corporation, the company that licensed the drug. M.W.D. is also a consultant for Siva Therapeutics and Kaio Therapy and a member of the Scientific Advisory Board of Innovate Biopharmaceuticals.
Glossary
- Pharmacokinetics
-
The time-dependent variation of drug concentrations in various compartments of the body following administration of a drug; often understood to refer to concentration in the blood plasma.
- Pharmacodynamics
-
The dependence of therapeutic effects on the level and time course of exposure to a drug; for cancer therapies, therapeutic effect is often described in terms of cell survival fraction or tumour growth inhibition.
- Enhanced permeability and retention (EPR) effect
-
The increased permeability of tumour microvessels relative to normal tissue vasculature, particularly in lipid and macromolecular agents and nanoparticles, leading to increased accumulation of such agents in the extravascular space in tumours. Enlarged gaps between endothelial cells occur where there is active angiogenesis. The gaps permit accumulation of nanoparticles within the tissue.
- Diffusivity
-
A physical parameter describing the rate of molecular diffusion of a solute in the presence of a concentration gradient; defined as the ratio of the diffusive flux to the spatial derivative of concentration.
- Hydraulic conductivity
-
A parameter defined as the ratio of the average fluid flow through the blood vessel wall per unit area divided by the net transmural (occurring across the entire vessel well) pressure driving filtration.
- Tumour cords
-
Cylindrical masses of tumour cells with a small blood vessel running centrally along their axes, appearing in pathological sections as structures with a central vessel and concentric ring of viable tumour cells. A typical radius of up to 100–150 μm corresponds to the maximal diffusion distance of oxygen in tissue. Beyond the oxygen diffusion distance, necrosis is often observed.
- Hypoxic regions
-
Tumour subregions where the partial pressure of oxygen (pO2) in tissue is >0 and <10 mmHg.
- First-pass metabolism
-
The reduction in the concentration of a drug in blood as a result of the initial metabolism of the drug in the liver before reaching the systemic circulatory system.
- Vascular endothelial growth factor
-
(VEGF). A cytokine (small protein) that potently stimulates blood vessel growth and increases vascular permeability.
- Tortuosity
-
Pertaining to a blood vessel; the presence of multiple bends and twists in the path of the vessel between its branch points as quantified by the ratio of the length of the vessel measured along its curved path to the straight-line distance between branch points. In tumours, this ratio tends to be greater than one, whereas in normal tissue, it is close to one.
- Vascular shunts
-
A relatively short, high-flow pathway from the arterial to the venous circulation. Identifiable shunt vessels are referred to as 'anatomical shunts', whereas shunting occurring because of abnormal network structure is referred to as 'functional shunting'.
- Vessel wall shear stress
-
The tangential force per unit area exerted on a solid boundary by a moving fluid such as blood.
- Stokes–Einstein radius
-
With reference to a solute, the radius of a hard sphere that diffuses at the same rate as the solute.
- Multicellular layer system
-
An experimental system in which tumour cells are allowed to proliferate on a semipermeable membrane until they reach a thickness of several cell layers. The membrane is placed between two reservoirs, and drugs or other solutes that are added to one reservoir pass to the other reservoir by diffusion across the multiple layers. Rates of diffusion and metabolism are estimated from the rates at which the concentrations vary in the reservoirs.
- Interstitial fluid pressure
-
(IFP). The hydrostatic pressure of the fluid that permeates the spaces between cells in a tissue; generally occurring as a result of fluid filtration from blood through the walls of blood vessels into the extravascular space.
- K trans
-
The rate constant describing the mass transfer between blood plasma and extravascular extracellular space per unit volume of tissue. This measurement is obtained using dynamic contrast-enhanced MRI.
- Vascular rarefaction
-
A decrease in the total length of blood vessels per unit volume of tissue.
- Dynamic contrast-enhanced MRI
-
(DCE-MRI). A method used to quantify the change in signal intensity in tissue over time after intravenous injection of an MRI contrast agent. The rates at which the contrast agent washes in and out of the tumour are used to estimate parameters related to perfusion and permeability of the microcirculation, including Ktrans.
- Texture analysis
-
A method used in image processing to assess the geometric arrangement of intensities within an image.
- T1 relaxivity
-
In MRI, T1 relaxation time is the time constant for aligned spins to decay to baseline after an MR pulse. Relaxivity is a measurement of how a contrast agent influences the T1 relaxation time. With calibration, relaxivity can be used to measure concentration of the contrast agent in tissue.
Rights and permissions
About this article
Cite this article
Dewhirst, M., Secomb, T. Transport of drugs from blood vessels to tumour tissue. Nat Rev Cancer 17, 738–750 (2017). https://doi.org/10.1038/nrc.2017.93
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc.2017.93
This article is cited by
-
Breaking through the basement membrane barrier to improve nanotherapeutic delivery to tumours
Nature Nanotechnology (2024)
-
Effect of vessel compression on blood flow in microvascular networks and its implications for tumour tissue hypoxia
Communications Physics (2024)
-
Spatially selective delivery of living magnetic microrobots through torque-focusing
Nature Communications (2024)
-
Quantitative imaging of doxorubicin diffusion and cellular uptake in biomimetic gels with human liver tumor cells
Drug Delivery and Translational Research (2024)
-
A carrier-free supramolecular nano-twin-drug for overcoming irinotecan-resistance and enhancing efficacy against colorectal cancer
Journal of Nanobiotechnology (2023)