Key Points
-
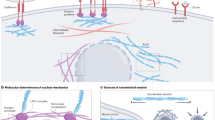
Engineers and physical scientists have pioneered research into understanding cancer as more than simply malignant cells with genetic mutations and instead as aberrant organs composed of cancer cells and their surrounding stroma, referred to as the tumour microenvironment (TME). Many aspects of the microenvironment are abnormal, which fuels tumour progression and treatment resistance.
-
Recent work using advanced in vivo imaging, computational modelling and animal models has identified barriers in the TME that hinder therapy and promote tumour progression.
-
Under pathological conditions, remodelling of the extracellular matrix (ECM) leads to fibre alignment, bundling and stiffening, which in turn alters tumour and stromal cell–matrix mechanics and interactions to enhance pro-angiogenic secretion from a range of cells in the TME as well as the migration of cancer cells. This promotes the invasion of tumour cells from the primary site into the circulation and the recruitment of endothelial cells for vascularization of the tumour to initiate tumour growth, invasion into the surrounding stroma and, finally, metastasis.
-
Tumour cells with a larger glycocalyx than normal cells exhibit extended gaps between the membrane and ECM, clustering of integrins, the exclusion of glycopolymers from regions of integrin adhesion and membrane bending. Engineered glycoprotein mimetics have been used to study how the physical properties of the glycocalyx coating alter cellular signalling and promote tumour survival and metastasis.
-
Drug delivery scientists pioneered the development of engineering systems that deliver therapeutics in a safe, effective and targeted fashion. Recent advances have focused on new delivery systems for cancer immunotherapy and gene therapy, as well as implantable devices for developing personalized medicine regimens.
-
Engineers and physical scientists have advanced imaging in oncology through the development of macroscopic imaging techniques in clinical settings, in addition to intravital optical techniques used in research settings that are increasingly used to detect various biomarkers. Clinical imaging probes developed by engineers and material scientists, such as fluorescent proteins, nanomaterials and labelled small and large molecules, have complemented these modalities.
-
Advances in microfluidics and microfabrication have led to the development of tissue and organ models that can incorporate physiological fluid flow and real-time optical imaging to study tumour cell migration and mechanotransduction. Microfluidics are also used to create human 'organs-on-chip' models for high-throughput drug screening, as well as isolation of rare circulating tumour cells and exosomes from patient blood samples.
Abstract
The principles of engineering and physics have been applied to oncology for nearly 50 years. Engineers and physical scientists have made contributions to all aspects of cancer biology, from quantitative understanding of tumour growth and progression to improved detection and treatment of cancer. Many early efforts focused on experimental and computational modelling of drug distribution, cell cycle kinetics and tumour growth dynamics. In the past decade, we have witnessed exponential growth at the interface of engineering, physics and oncology that has been fuelled by advances in fields including materials science, microfabrication, nanomedicine, microfluidics, imaging, and catalysed by new programmes at the National Institutes of Health (NIH), including the National Institute of Biomedical Imaging and Bioengineering (NIBIB), Physical Sciences in Oncology, and the National Cancer Institute (NCI) Alliance for Nanotechnology. Here, we review the advances made at the interface of engineering and physical sciences and oncology in four important areas: the physical microenvironment of the tumour and technological advances in drug delivery; cellular and molecular imaging; and microfluidics and microfabrication. We discussthe research advances, opportunities and challenges for integrating engineering and physical sciences with oncology to develop new methods to study, detect and treat cancer, and we also describe the future outlook for these emerging areas.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Jain, R. K., Martin, J. D. & Stylianopoulos, T. The role of mechanical forces in tumor growth and therapy. Annu. Rev. Biomed. Eng. 16, 321–346 (2014). Comprehensive review of the role of physical forces in tumour progression and therapy for those new to the fields of engineering and physical sciences in oncology.
Wirtz, D., Konstantopoulos, K. & Searson, P. C. The physics of cancer: the role of physical interactions and mechanical forces in metastasis. Nat. Rev. Cancer 11, 512–522 (2011).
Lu, P., Weaver, V. M. & Werb, Z. The extracellular matrix: a dynamic niche in cancer progression. J. Cell Biol. 196, 395–406 (2012).
Tse, J. M. et al. Mechanical compression drives cancer cells toward invasive phenotype. Proc. Natl Acad. Sci. USA 109, 911–916 (2012).
Jain, R. K. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell 26, 605–622 (2014).
Jain, R. K. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J. Clin. Oncol. 31, 2205–2218 (2013).
Mitchell, M. J. & King, M. R. Computational and experimental models of cancer cell response to fluid shear stress. Frontiers Oncol. 3, 44 (2013).
DeVita, V. T. & Chu, E. A. History of cancer chemotherapy. Cancer Res. 68, 8643–8653 (2008).
Verellen, D. et al. Innovations in image-guided radiotherapy. Nat. Rev. Cancer 7, 949–960 (2007).
Winkler, F. et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell 6, 553–563 (2004). Seminal study revealing the molecular and physiological mechanisms of vascular normalization, along with how normalization improves the outcome of various therapies.
Wong, C. et al. Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc. Natl Acad. Sci. USA 108, 2426–2431 (2011).
Jain, R. K. An indirect way to tame cancer. Scientif. Am. 310, 46–53 (2014).
Jain, R. K. & Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 7, 653–664 (2010).
Chauhan, V. P. et al. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat. Nanotechnol. 7, 383–388 (2012). First study demonstrating that normalization of leaky, disordered tumour vasculature enhances the delivery of smaller nanoparticle therapeutics to tumours.
Bhatia, S. N. & Ingber, D. E. Microfluidic organs-on-chips. Nat. Biotechnol. 32, 760–772 (2014).
Esch, M. B., King, T. L. & Shuler, M. L. The role of body-on-a-chip devices in drug and toxicity studies. Annu. Rev. Biomed. Eng. 13, 55–72 (2011).
Swartz, M. A. & Lund, A. W. Lymphatic and interstitial flow in the tumour microenvironment: linking mechanobiology with immunity. Nat. Rev. Cancer 12, 210–219 (2012).
Hanahan, D. & Coussens, L. M. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21, 309–322 (2012).
Gajewski, T. F., Schreiber, H. & Fu, Y.-X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 14, 1014–1022 (2013).
Jain, R. K., Munn, L. L. & Fukumura, D. Dissecting tumour pathophysiology using intravital microscopy. Nat. Rev. Cancer 2, 266–276 (2002).
Correia, A. L. & Bissell, M. J. The tumor microenvironment is a dominant force in multidrug resistance. Drug Resist. Updat. 15, 39–49 (2012).
Trédan, O., Galmarini, C. M., Patel, K. & Tannock, I. F. Drug resistance and the solid tumor microenvironment. J. Natl Cancer Inst. 99, 1441–1454 (2007).
Chauhan, V. P. & Jain, R. K. Strategies for advancing cancer nanomedicine. Nat. Mater. 12, 958–962 (2013).
Wilhelm, S. et al. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 1, 16014 (2016).
Matsumura, Y. & Maeda, H. A. New concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 46, 6387–6392 (1986).
Gerlowski, L. E. & Jain, R. K. Microvascular permeability of normal and neoplastic tissues. Microvasc. Res. 31, 288–305 (1986).
Dvorak, H. F., Brown, L. F., Detmar, M. & Dvorak, A. M. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am. J. Pathol. 146, 1029–1039 (1995).
Carmeliet, P. & Jain, R. K. Molecular mechanisms and clinical applications of angiogenesis. Nature 473, 298–307 (2011).
Padera, T. P. et al. Cancer cells compress intratumour vessels. Nature 427, 695–695 (2004).
Bertrand, N., Wu, J., Xu, X., Kamaly, N. & Farokhzad, O. C. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 66, 2–25 (2014).
Koukourakis, M. I. et al. Liposomal doxorubicin and conventionally fractionated radiotherapy in the treatment of locally advanced non-small-cell lung cancer and head and neck cancer. J. Clin. Oncol. 17, 3512–3521 (1999).
Stylianopoulos, T. & Jain, R. K. Design considerations for nanotherapeutics in oncology. Nanomedicine 11, 1893–1907 (2015).
Jain, R. K. & Baxter, L. T. Mechanisms of heterogeneous distribution of monoclonal antibodies and other macromolecules in tumors: significance of elevated interstitial pressure. Cancer Res. 48, 7022–7032 (1988). Seminal paper on the role of elevated IFP as a barrier to drug delivery.
Tong, R. T. et al. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 64, 3731–3736 (2004).
Batchelor, T. T. et al. Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. Proc. Natl Acad. Sci. USA 110, 19059–19064 (2013). Clinical evidence that vascular normalization and the resulting increase in perfusion improve survival in cancer patients.
Heist, R. S. et al. Improved tumor vascularization after anti-VEGF therapy with carboplatin and nab-paclitaxel associates with survival in lung cancer. Proc. Natl Acad. Sci. USA 112, 1547–1552 (2015).
Tolaney, S. M. et al. Role of vascular density and normalization in response to neoadjuvant bevacizumab and chemotherapy in breast cancer patients. Proc. Natl Acad. Sci. USA 112, 14325–14330 (2015).
US National Library of Medicine. Clinicaltrials.govhttps://www.clinicaltrials.gov/ct2/show/NCT00662506 (2017).
US National Library of Medicine. Clinicaltrials.govhttps://www.clinicaltrials.gov/ct2/show/NCT00642759 (2017).
Chauhan, V. P. et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat. Commun. 4, 2516 (2013).
Stylianopoulos, T. et al. Causes, consequences, and remedies for growth-induced solid stress in murine and human tumors. Proc. Natl Acad. Sci. USA 109, 15101–15108 (2012).
Nia, H. T. et al. Solid stress and elastic energy as measures of tumour mechanopathology. Nat. Biomed. Eng. 1, 0004 (2016).
Incio, J. et al. Obesity-induced inflammation and desmoplasia promote pancreatic cancer progression and resistance to chemotherapy. Cancer Discov. 6, 852–869 (2016). Study demonstrating the effects of obesity on tumour mechanics, along with potential strategies to overcome these effects using clinically available antifibrotic and inflammatory agents.
Liu, H. et al. Use of angiotensin system inhibitors is associated with immune activation and longer survival in non-metastatic pancreatic ductal adenocarcinoma. Clin. Cancer Res. http://dx.doi.org/10.1158/1078-0432.CCR-17-0256 (2017).
Murphy, J. E. et al. TGF-B1 inhibition with losartan in combination with FOLFIRINOX (F-NOX) in locally advanced pancreatic cancer (LAPC): preliminary feasibility and R0 resection rates from a prospective phase II study. J. Clin. Oncol. 35, 386–386 (2017).
US National Library of Medicine. Clinicaltrials.govhttps://clinicaltrials.gov/show/NCT01821729 (2017).
Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 16, 582–598 (2016).
Provenzano, P. P. et al. Collagen density promotes mammary tumor initiation and progression. BMC Med. 6, 11 (2008).
Levental, K. R. et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891–906 (2009).
Naba, A., Clauser, K. R., Lamar, J. M., Carr, S. A. & Hynes, R. O. Extracellular matrix signatures of human mammary carcinoma identify novel metastasis promoters. eLife 3, e01308 (2014).
Provenzano, P. P. et al. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 4, 38 (2006).
Rivron, N. C. et al. Tissue deformation spatially modulates VEGF signaling and angiogenesis. Proc. Natl Acad. Sci. USA 109, 6886–6891 (2012).
Schrader, J. et al. Matrix stiffness modulates proliferation, chemotherapeutic response, and dormancy in hepatocellular carcinoma cells. Hepatology 53, 1192–1205 (2011).
Youk, J. H. et al. Comparison of strain and shear wave elastography for the differentiation of benign from malignant breast lesions, combined with B-mode ultrasonography: qualitative and quantitative assessments. Ultrasound Med. Biol. 40, 2336–2344 (2014).
Cheng, G., Tse, J., Jain, R. K. & Munn, L. L. Micro-environmental mechanical stress controls tumor spheroid size and morphology by suppressing proliferation and inducing apoptosis in cancer cells. PLoS ONE 4, e4632 (2009).
Calvo, F. et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat. Cell Biol. 15, 637–646 (2013).
Paszek, M. J. et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 8, 241–254 (2005). Seminal work demonstrating that elevated tissue stiffness promotes malignant behaviour via modulation of integrins.
Wang, K. et al. Stiffening and unfolding of early deposited-fibronectin increase proangiogenic factor secretion by breast cancer-associated stromal cells. Biomaterials 54, 63–71 (2015).
Artym, V. V. et al. Dense fibrillar collagen is a potent inducer of invadopodia via a specific signaling network. J. Cell Biol. 208, 331–350 (2015).
Doyle, A. D., Carvajal, N., Jin, A., Matsumoto, K. & Yamada, K. M. Local 3D matrix microenvironment regulates cell migration through spatiotemporal dynamics of contractility-dependent adhesions. Nat. Commun. 6, 8720 (2015).
Engler, A. J., Sen, S., Sweeney, H. L. & Discher, D. E. Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006). Seminal study demonstrating that stem cells that generate specialized cells within the body take cues from tissue stiffness to specify lineage and commit to phenotypes.
Bordeleau, F. et al. Matrix stiffening promotes a tumor vasculature phenotype. Proc. Natl Acad. Sci. USA 114, 492–497 (2017).
Hynes, R. O. The extracellular matrix: not just pretty fibrils. Science 326, 1216–1219 (2009).
Ulrich, T. A., De- Juan-Pardo, E. M. & Kumar, S. The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells. Cancer Res. 69, 4167–4174 (2009).
Weigelin, B., Bakker, G.-J. & Friedl, P. Intravital third harmonic generation microscopy of collective melanoma cell invasion. IntraVital 1, 32–43 (2012).
Blehm, B. H., Jiang, N., Kotobuki, Y. & Tanner, K. Deconstructing the role of the ECM microenvironment on drug efficacy targeting MAPK signaling in a pre-clinical platform for cutaneous melanoma. Biomaterials 56, 129–139 (2015).
Seo, B. R. et al. Obesity-dependent changes in interstitial ECM mechanics promote breast tumorigenesis. Sci. Transl Med. 7, 301ra130 (2015). Study linking obesity, fibrotic remodelling and ECM mechanics to breast tumorigenesis.
Fukumura, D., Incio, J., Shankaraiah, R. C. & Jain, R. K. Obesity and cancer: an angiogenic and inflammatory link. Microcirculation 23, 191–206 (2016).
Laklai, H. et al. Genotype tunes pancreatic ductal adenocarcinoma tissue tension to induce matricellular fibrosis and tumor progression. Nat. Med. 22, 497–505 (2016).
Bailey, J. M. et al. Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clin. Cancer Res. 14, 5995–6004 (2008).
Olive, K. P. et al. Inhibition of hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324, 1457–1461 (2009).
Rhim, A. D. et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 25, 735–747 (2014).
Özdemir, B. C. et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 25, 719–734 (2014).
Sherman, M. H. et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 159, 80–93 (2014).
Pinho, S. S. & Reis, C. A. Glycosylation in cancer: mechanisms and clinical implications. Nat. Rev. Cancer 15, 540–555 (2015).
Mitchell, M. J. & King, M. R. Physical Biology in Cancer. 3. The role of cell glycocalyx in vascular transport of circulating tumor cells. Am. J. Physiol., Cell Physiol. 306, C89–C97 (2014).
Weinbaum, S., Tarbell, J. M. & Damiano, E. R. The structure and function of the endothelial glycocalyx layer. Annu. Rev. Biomed. Eng. 9, 121–167 (2007).
Hollingsworth, M. A. & Swanson, B. J. Mucins in cancer: protection and control of the cell surface. Nat. Rev. Cancer 4, 45–60 (2004).
Itano, N., Sawai, T., Miyaishi, O. & Kimata, K. Relationship between hyaluronan production and metastatic potential of mouse mammary carcinoma cells. Cancer Res. 59, 2499–2504 (1999).
Rahbari, N. N. et al. Anti-VEGF therapy induces ECM remodeling and mechanical barriers to therapy in colorectal cancer liver metastases. Sci. Transl Med. 8, 360ra135 (2016).
Paszek, M. J. et al. The cancer glycocalyx mechanically primes integrin-mediated growth and survival. Nature 511, 319–325 (2014). First study implicating the bulky glycocalyx of tumour cells as a feature that promotes metastasis via mechanically enhanced cell-surface-receptor function.
Hudak, J. E., Canham, S. M. & Bertozzi, C. R. Glycocalyx engineering reveals a Siglec-based mechanism for NK cell immunoevasion. Nature Chem. Biol. 10, 69–75 (2013).
Läubli, H. et al. Engagement of myelomonocytic Siglecs by tumor-associated ligands modulates the innate immune response to cancer. Proc. Natl Acad. Sci. USA 111, 14211–14216 (2014).
Xiao, H., Woods, E. C., Vukojicic, P. & Bertozzi, C. R. Precision glycocalyx editing as a strategy for cancer immunotherapy. Proc. Natl Acad. Sci. USA 113, 10304–10309 (2016).
Langer, R. & Folkman, J. Polymers for the sustained release of proteins and other macromolecules. Nature 263, 797–800 (1976). First demonstration of the use of polymeric materials for controlled, sustained release of high-molecular-weight compounds for >100 days.
Yatvin, M. B., Weinstein, J. N., Dennis, W. H. & Blumenthal, R. Design of liposomes for enhanced local release of drugs by hyperthermia. Science 202, 1290–1293 (1978).
Brownlee, M. & Cerami, A. A glucose-controlled insulin-delivery system: semisynthetic insulin bound to lectin. Science 206, 1190–1191 (1979).
Steichen, S. D., Caldorera-Moore, M. & Peppas, N. A. A review of current nanoparticle and targeting moieties for the delivery of cancer therapeutics. Eur. J. Pharm. Sci. 48, 416–427 (2013).
Langer, R. Drug delivery and targeting. Nature 392, 5–10 (1998).
Sampath, P. & Brem, H. Implantable slow-release chemotherapeutic polymers for the treatment of malignant brain tumors. Cancer Control 5, 130–137 (1998).
Okada, H., Doken, Y., Ogawa, Y. & Toguchi, H. Preparation of three-month depot injectable microspheres of leuprorelin acetate using biodegradable polymers. Pharm. Res. 11, 1143–1147 (1994).
Gabizon, A. et al. Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Res. 54, 987–992 (1994). Seminal report showing prolonged circulation time and enhanced tumour accumulation of polyethylene glycol-coated doxorubicin liposome formulations (Doxil) in patients, compared with free doxorubicin.
Gu, L. & Mooney, D. J. Biomaterials and emerging anticancer therapeutics: engineering the microenvironment. Nat. Rev. Cancer 16, 56–66 (2016).
Moon, J. J., Huang, B. & Irvine, D. J. Engineering nano- and microparticles to tune immunity. Adv. Mater. 24, 3724–3746 (2012).
Liu, H. et al. Structure-based programming of lymph-node targeting in molecular vaccines. Nature 507, 519–522 (2014). Engineering of an amphiphilic cancer vaccine that 'hitchhikes' with serum albumin to traffic efficiently to lymph nodes, enabling substantially increased potency and safety of subunit vaccines.
Jeanbart, L. et al. Enhancing efficacy of anticancer vaccines by targeted delivery to tumor-draining lymph nodes. Cancer Immunol. Res. 2, 436–447 (2014).
Thomas, S. N., Vokali, E., Lund, A. W., Hubbell, J. A. & Swartz, M. A. Targeting the tumor-draining lymph node with adjuvanted nanoparticles reshapes the anti-tumor immune response. Biomaterials 35, 814–824 (2014).
Kranz, L. M. et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 534, 396–401 (2016). Synthesis of a nanoparticle RNA vaccine that targets dendritic cells after systemic administration, leading to an antitumour immune response with antiviral features as well as potential efficacy in patients with advanced melanoma.
US National Library of Medicine. Clinicaltrials.govhttps://clinicaltrials.gov/ct2/show/NCT02410733 (2016).
Chahal, J. S. et al. Dendrimer-RNA nanoparticles generate protective immunity against lethal Ebola, H1N1 influenza, and Toxoplasma gondii challenges with a single dose. Proc. Natl Acad. Sci. USA 113, E4133–E4142 (2016).
Restifo, N. P., Dudley, M. E. & Rosenberg, S. A. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat. Rev. Immunol. 12, 269–281 (2012).
Stephan, M. T., Moon, J. J., Um, S. H., Bershteyn, A. & Irvine, D. J. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat. Med. 16, 1035–1041 (2010).
Stephan, M. T., Stephan, S. B., Bak, P., Chen, J. & Irvine, D. J. Synapse-directed delivery of immunomodulators using T-cell-conjugated nanoparticles. Biomaterials 33, 5776–5787 (2012).
Huang, B. et al. Active targeting of chemotherapy to disseminated tumors using nanoparticle-carrying T cells. Sci. Transl Med. 7, 291ra94 (2015).
Mitchell, M. J., Wayne, E., Rana, K., Schaffer, C. B. & King, M. R. TRAIL-coated leukocytes that kill cancer cells in the circulation. Proc. Natl Acad. Sci. USA 111, 930–935 (2014).
Mitchell, M. J. & King, M. R. Leukocytes as carriers for targeted cancer drug delivery. Expert Opin. Drug Deliv. 12, 375–392 (2014).
Mitchell, M. J. et al. Polymeric mechanical amplifiers of immune cytokine-mediated apoptosis. Nat. Commun. 8, 14179 (2017).
Wayne, E. C. et al. TRAIL-coated leukocytes that prevent the bloodborne metastasis of prostate cancer. J. Control. Release 223, 215–223 (2016).
Zuckerman, J. E. & Davis, M. E. Clinical experiences with systemically administered siRNA-based therapeutics in cancer. Nat. Rev. Drug Discov. 14, 843–856 (2015).
US National Library of Medicine. Clinicaltrials.govhttps://clinicaltrials.gov/ct2/show/NCT01960348 (2017).
Davis, M. E. et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature 464, 1067–1070 (2010).
US National Library of Medicine. Clinicaltrials.govhttps://clinicaltrials.gov/ct2/show/NCT00938574 (2013).
US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT01591356 (2017).
Yin, H. et al. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 15, 541–555 (2014).
Zhu, X. et al. Long-circulating siRNA nanoparticles for validating Prohibitin1-targeted non-small cell lung cancer treatment. Proc. Natl Acad. Sci. USA 112, 7779–7784 (2015).
Ren, Y. et al. Targeted tumor-penetrating siRNA nanocomplexes for credentialing the ovarian cancer oncogene ID4. Sci. Transl Med. 4, 147ra112 (2012).
Jensen, S. A. et al. Spherical nucleic acid nanoparticle conjugates as an RNAi-based therapy for glioblastoma. Sci. Transl Med. 5, 209ra152 (2013).
Dahlman, J. E. et al. In vivo endothelial siRNA delivery using polymeric nanoparticles with low molecular weight. Nat. Nanotechnol. 9, 648–655 (2014).
Akinc, A. et al. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol. Ther. 18, 1357–1364 (2010).
Xue, W. et al. Small RNA combination therapy for lung cancer. Proc. Natl Acad. Sci. USA 111, E3553–E3561 (2014).
Deng, Z. J. et al. Layer-by-layer nanoparticles for systemic codelivery of an anticancer drug and siRNA for potential triple-negative breast cancer treatment. ACS Nano 7, 9571–9584 (2013).
Zhao, Y. et al. PolyMetformin combines carrier and anticancer activities for in vivo siRNA delivery. Nat. Commun. 7, 11822 (2016).
Coombes, R. C. Drug testing in the patient: toward personalized cancer treatment. Sci. Transl Med. 7, 284ps10 (2015).
Jonas, O. et al. An implantable microdevice to perform high-throughput in vivo drug sensitivity testing in tumors. Sci. Transl Med. 7, 284ra57 (2015). Development of a minimally invasive microdevice that can be implanted and removed from tumours using a biopsy needle, enabling direct drug sensitivity testing of many compounds within a patient.
Klinghoffer, R. A. et al. A technology platform to assess multiple cancer agents simultaneously within a patient's tumor. Sci. Transl Med. 7, 284ra58 (2015).
US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT01831505 (2017).
US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT03056599 (2017).
Yin, H., Kauffman, K. J. & Anderson, D. G. Delivery technologies for genome editing. Nat. Rev. Drug Discov. 16, 387–399 (2017).
Weissleder, R. & Nahrendorf, M. Advancing biomedical imaging. Proc. Natl Acad. Sci. USA 112, 14424–14428 (2015).
Bao, G., Mitragotri, S. & Tong, S. Multifunctional nanoparticles for drug delivery and molecular imaging. Annu. Rev. Biomed. Eng. 15, 253–282 (2013).
Rimm, D. L. What brown cannot do for you. Nat. Biotechnol. 24, 914–916 (2006).
Angelo, M. et al. Multiplexed ion beam imaging of human breast tumors. Nat. Med. 20, 436–442 (2014). Development of a new technique — multiplex ion beam imaging — which utilizes secondary ion mass spectrometry and antibodies labelled with isotopically pure elemental metals to detect up to 100 tumour antigens within a single sample.
Bendall, S. C. et al. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science 332, 687–696 (2011).
Peet, A. C., Arvanitis, T. N., Leach, M. O. & Waldman, A. D. Functional imaging in adult and paediatric brain tumours. Nat. Rev. Clin. Oncol. 9, 700–711 (2012).
Choi, C. et al. 2-Hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat. Med. 18, 624–629 (2012).
Zackrisson, S., van de Ven, S. M. W. Y. & Gambhir, S. S. Light in and sound out: emerging translational strategies for photoacoustic imaging. Cancer Res. 74, 979–1004 (2014).
Jokerst, J. V., Cole, A. J., Van de Sompel, D. & Gambhir, S. S. Gold nanorods for ovarian cancer detection with photoacoustic imaging and resection guidance via Raman imaging in living mice. ACS Nano 6, 10366–10377 (2012).
la Zerda, de, A. et al. Family of enhanced photoacoustic imaging agents for high-sensitivity and multiplexing studies in living mice. ACS Nano 6, 4694–4701 (2012).
Kircher, M. F. et al. A brain tumor molecular imaging strategy using a new triple-modality MRI-photoacoustic-Raman nanoparticle. Nat. Med. 18, 829–834 (2012).
Pu, K. et al. Semiconducting polymer nanoparticles as photoacoustic molecular imaging probes in living mice. Nat. Nanotechnol. 9, 233–239 (2014).
Jokerst, J. V., Lobovkina, T., Zare, R. N. & Gambhir, S. S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine 6, 715–728 (2011).
Gu, F. et al. Precise engineering of targeted nanoparticles by using self-assembled biointegrated block copolymers. Proc. Natl Acad. Sci. USA 105, 2586–2591 (2008).
Ruoslahti, E., Bhatia, S. N. & Sailor, M. J. Targeting of drugs and nanoparticles to tumors. J. Cell Biol. 188, 759–768 (2010).
Ghosh, D. et al. M13-templated magnetic nanoparticles for targeted in vivo imaging of prostate cancer. Nat. Nanotechnology 7, 677–682 (2012).
Weissleder, R., Schwaiger, M. C., Gambhir, S. S. & Hricak, H. Imaging approaches to optimize molecular therapies. Sci. Transl Med. 8, 355ps16 (2016).
Miller, M. A. & Weissleder, R. Imaging the pharmacology of nanomaterials by intravital microscopy: toward understanding their biological behavior. Adv. Drug Deliv. Rev. 113, 61–86 (2016).
Thurber, G. M. et al. Single-cell and subcellular pharmacokinetic imaging allows insight into drug action in vivo. Nat. Commun. 4, 1504 (2013).
Laughney, A. M. et al. Single-cell pharmacokinetic imaging reveals a therapeutic strategy to overcome drug resistance to the microtubule inhibitor eribulin. Sci. Transl Med. 6, 261ra152 (2014).
Miller, M. A., Askevold, B., Yang, K. S., Kohler, R. H. & Weissleder, R. Platinum compounds for high-resolution in vivo cancer imaging. ChemMedChem 9, 1131–1135 (2014).
Dubach, J. M. et al. In vivo imaging of specific drug-target binding at subcellular resolution. Nat. Commun. 5, 3946 (2014).
Miller, M. A. et al. Tumour-associated macrophages act as a slow-release reservoir of nano-therapeutic Pt(IV) pro-drug. Nat. Commun. 6, 8692 (2015).
Miller, M. A. et al. Predicting therapeutic nanomedicine efficacy using a companion magnetic resonance imaging nanoparticle. Sci. Transl Med. 7, 314ra183 (2015). A combined approach of MRI and intravital imaging, which demonstrated that magnetic nanoparticles can be used to select for tumours in vivo with high EPR and thus are more likely to respond to treatment with therapeutic nanoparticles.
Harisinghani, M. G. et al. A pilot study of lymphotrophic nanoparticle-enhanced magnetic resonance imaging technique in early stage testicular cancer: a new method for noninvasive lymph node evaluation. Urology 66, 1066–1071 (2005).
Wyckoff, J., Gligorijevic, B., Entenberg, D., Segall, J. & Condeelis, J. High-resolution multiphoton imaging of tumors in vivo. Cold Spring Harb. Protoc. 2011, 1167–1184 (2011).
Jain, R. K. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat. Med. 7, 987–989 (2001). Seminal paper to put forward the vascular normalization hypothesis that changed the paradigm in antiangiogenic therapy.
Wang, W. et al. Coordinated regulation of pathways for enhanced cell motility and chemotaxis is conserved in rat and mouse mammary tumors. Cancer Res. 67, 3505–3511 (2007).
Wyckoff, J. et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 64, 7022–7029 (2004).
Lohela, M. et al. Intravital imaging reveals distinct responses of depleting dynamic tumor-associated macrophage and dendritic cell subpopulations. Proc. Natl Acad. Sci. USA 111, E5086–E5095 (2014).
Goswami, S. et al. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res. 65, 5278–5283 (2005).
Wyckoff, J. B. et al. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 67, 2649–2656 (2007).
Peterson, T. E. et al. Dual inhibition of Ang-2 and VEGF receptors normalizes tumor vasculature and prolongs survival in glioblastoma by altering macrophages. Proc. Natl Acad. Sci. USA 113, 4470–4475 (2016).
Brown, E. et al. Dynamic imaging of collagen and its modulation in tumors in vivo using second-harmonic generation. Nat. Med. 9, 796–800 (2003).
Nadiarnykh, O., LaComb, R. B., Brewer, M. A. & Campagnola, P. J. Alterations of the extracellular matrix in ovarian cancer studied by Second Harmonic Generation imaging microscopy. BMC Cancer 10, 94 (2010).
Conklin, M. W. et al. Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am. J. Pathol. 178, 1221–1232 (2011).
Gailhouste, L. et al. Fibrillar collagen scoring by second harmonic microscopy: a new tool in the assessment of liver fibrosis. J. Hepatol. 52, 398–406 (2010).
Weigelin, B., Bakker, G.-J. & Friedl, P. Third harmonic generation microscopy of cells and tissue organization. J. Cell Sci. 129, 245–255 (2016).
Lambert, A. W., Pattabiraman, D. R. & Weinberg, R. A. Emerging biological principles of metastasis. Cell 168, 670–691 (2017).
Bruns, O. T. et al. Next-generation in vivo optical imaging with short-wave infrared quantum dots. Nat. Biomed. Eng. 1, 0056 (2017).
Errico, C. et al. Ultrafast ultrasound localization microscopy for deep super-resolution vascular imaging. Nature 527, 499–502 (2015).
Nelson, S. J. et al. Metabolic imaging of patients with prostate cancer using hyperpolarized [1-13C]pyruvate. Sci. Transl Med. 5, 198ra108 (2013).
Walker-Samuel, S. et al. In vivo imaging of glucose uptake and metabolism in tumors. Nat. Med. 19, 1067–1072 (2013).
Shields, J. D. et al. Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell 11, 526–538 (2007). Seminal study identifying a novel mechanism underlying metastasis through the lymphatic system, named autologous chemotaxis, which directs tumour cell migration via autocrine chemokine gradients induced by interstitial flow.
Polacheck, W. J., Charest, J. L. & Kamm, R. D. Interstitial flow influences direction of tumor cell migration through competing mechanisms. Proc. Natl Acad. Sci. USA 108, 11115–11120 (2011).
Paul, C. D., Mistriotis, P. & Konstantopoulos, K. Cancer cell motility: lessons from migration in confined spaces. Nat. Rev. Cancer 17, 131–140 (2016).
Mekhdjian, A. H. et al. Integrin-mediated traction force enhances paxillin molecular associations and adhesion dynamics that increase the invasiveness of tumor cells into a three-dimensional extracellular matrix. Mol. Biol. Cell 28, 1467–1488 (2017).
Alexander, S., Weigelin, B., Winkler, F. & Friedl, P. Preclinical intravital microscopy of the tumour-stroma interface: invasion, metastasis, and therapy response. Curr. Opin. Cell Biol. 25, 659–671 (2013).
Kraning-Rush, C. M., Carey, S. P. & Lampi, M. C. Microfabricated collagen tracks facilitate single cell metastatic invasion in 3D. Integr. Biol. 5, 606–616 (2013).
Carey, S. P. et al. Comparative mechanisms of cancer cell migration through 3D matrix and physiological microtracks. Am. J. Physiol., Cell Physiol. 308, C436–C447 (2015).
Pathak, A. & Kumar, S. Independent regulation of tumor cell migration by matrix stiffness and confinement. Proc. Natl Acad. Sci. 109, 10334–10339 (2012).
Stroka, K. M. et al. Water permeation drives tumor cell migration in confined microenvironments. Cell 157, 611–623 (2014).
Raab, M. et al. ESCRT III repairs nuclear envelope ruptures during cell migration to limit DNA damage and cell death. Science 352, 359–362 (2016).
Denais, C. M. et al. Nuclear envelope rupture and repair during cancer cell migration. Science 352, 353–358 (2016).
Irianto, J. et al. DNA damage follows repair factor depletion and portends genome variation in cancer cells after pore migration. Curr. Biol. 27, 210–223 (2017).
Irianto, J. et al. Nuclear constriction segregates mobile nuclear proteins away from chromatin. Mol. Biol. Cell 27, 4011–4020 (2016).
Esch, E. W., Bahinski, A. & Huh, D. Organs-on-chips at the frontiers of drug discovery. Nat. Rev. Drug Discov. 14, 248–260 (2015).
Song, J. W. et al. Microfluidic endothelium for studying the intravascular adhesion of metastatic breast cancer cells. PLoS ONE 4, e5756 (2009).
Bersini, S. et al. A microfluidic 3D in vitro model for specificity of breast cancer metastasis to bone. Biomaterials 35, 2454–2461 (2013).
Sung, J. H. & Shuler, M. L. A micro cell culture analog (μCCA) with 3D hydrogel culture of multiple cell lines to assess metabolism-dependent cytotoxicity of anti-cancer drugs. Lab Chip 9, 1385–1394 (2009). Early work using organs-on-chip models, demonstrating that microfluidic devices combined with 3D hydrogel cell cultures representing individual organs can accurately reproduce anticancer drug effects observed in vivo.
Sung, J. H., Kam, C. & Shuler, M. L. A microfluidic device for a pharmacokinetic-pharmacodynamic (PK-PD) model on a chip. Lab Chip 10, 446–455 (2010).
Yu, M., Stott, S., Toner, M., Maheswaran, S. & Haber, D. A. Circulating tumor cells: approaches to isolation and characterization. J. Cell Biol. 192, 373–382 (2011).
Yu, M. et al. Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science 345, 216–220 (2014).
Murlidhar, V., Rivera- Báez, L. & Nagrath, S. Affinity versus label-free isolation of circulating tumor cells: who wins? Small 12, 4450–4463 (2016).
Nagrath, S. et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 450, 1235–1239 (2007). Seminal study that developed a unique flow-based microfluidic platform containing antibody-coated microposts to isolate rare CTCs from the blood of patients with metastatic cancer.
Yoon, H. J. et al. Sensitive capture of circulating tumour cells by functionalized graphene oxide nanosheets. Nat. Nanotechnol. 8, 735–741 (2013).
Gleghorn, J. P. et al. Capture of circulating tumor cells from whole blood of prostate cancer patients using geometrically enhanced differential immunocapture (GEDI) and a prostate-specific antibody. Lab Chip 10, 27–29 (2010).
Ozkumur, E. et al. Inertial focusing for tumor antigen-dependent and -independent sorting of rare circulating tumor cells. Sci. Transl Med. 5, 179ra47 (2013).
Hou, J.-M. et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small cell lung cancer. J. Clin. Oncol. 30, 525–532 (2012).
Cheung, K. J. & Ewald, A. J. A collective route to metastasis: seeding by tumor cell clusters. Science 352, 167–169 (2016).
Cheung, K. J. et al. Polyclonal breast cancer metastases arise from collective dissemination of keratin 14- expressing tumor cell clusters. Proc. Natl Acad. Sci. USA 113, E854–E863 (2016).
Sarioglu, A. F. et al. A microfluidic device for label-free, physical capture of circulating tumor cell clusters. Nat. Methods 12, 685–691 (2015).
Hoshino, A. et al. Tumour exosome integrins determine organotropic metastasis. Nature 527, 329–335 (2015).
Liga, A., Vliegenthart, A., Oosthuyzen, W., Dear, J. W. & Kersaudy-Kerhoas, M. Exosome isolation: a microfluidic road-map. Lab Chip 15, 2388–2394 (2015).
Chen, C. et al. Microfluidic isolation and transcriptome analysis of serum microvesicles. Lab Chip 10, 505–511 (2010).
Im, H. et al. Label-free detection and molecular profiling of exosomes with a nano-plasmonic sensor. Nat. Biotechnol. 32, 490–495 (2014).
Wunsch, B. H. et al. Nanoscale lateral displacement arrays for the separation of exosomes and colloids down to 20 nm. Nat. Nanotechnol. 11, 936–940 (2016).
Farrell, D. et al. Recent advances from the National Cancer Institute Alliance for Nanotechnology in Cancer. ACS Nano 4, 589–594 (2010).
Kuhn, N. Z. & Nagahara, L. A. Integrating physical sciences perspectives in cancer research. Sci. Transl Med. 5, 183fs14 (2013).
Sharp, P. & Hockfield, S. Convergence: the future of health. Science 355, 589–589 (2017).
Ewald, A. J. & Egeblad, M. Cancer: sugar-coated cell signalling. Nature 511, 298–299 (2014).
De Vries, J. & Figdor, C. Immunotherapy: cancer vaccine triggers antiviral-type defences. Nature 534, 329–331 (2016).
Speicher, M. R. & Pantel, K. Tumor signatures in the blood. Nat. Biotechnol. 32, 441–443 (2014).
Acknowledgements
This work was supported in part by a Cancer Center Support (core) Grant P30-CA14051 from the National Cancer Institute and a grant from the Koch Institute's Marble Centre for Cancer Nanomedicine (to R.L.) and the National Cancer Institute (P01-CA080124, R01-CA126642, R01-CA115767, R01-CA096915, R01-CA085140, R01-CA098706) and NCI Outstanding Investigator Award (R35-CA197743) (to R.K.J.). M.J.M. was supported by a Burroughs Wellcome Fund Career Award at the Scientific Interface, an NIH F32 fellowship (award number CA200351) and a grant from the Burroughs Wellcome Fund (no. 1015145). The authors thank V. Chauhan, M. Oberli, K. Kozielski, K. Wang, B. R. Seo, D. Fukumura, L. Munn and T. Stylianopoulos for helpful discussions and feedback on the manuscript. The authors thank K. Wang for assisting with conceptualization of figures.
Author information
Authors and Affiliations
Contributions
M.J.M., R.K.J. and R.L. conceived the ideas, researched the data for the manuscript, discussed the manuscript content and wrote the manuscript. M.J.M. designed the display items. All authors reviewed and edited the article before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Tumour microenvironment
-
(TME). The microenvironment surrounding cancer cells, which is composed of blood and lymphatic vessels, fibroblasts, immune cells and other non-malignant host cells, all embedded within extracellular matrix.
- Interstitial fluid pressure
-
(IFP). Pressure exerted by free interstitial tissue fluid. Increased IFP in tumours pushes fluid, growth factors, administered therapeutic molecules and cells to the peri-tumour tissue, aiding tumour progression.
- Enhanced permeability and retention
-
(EPR). An effect based on proposed mechanisms for selective tumour delivery of drugs. These mechanisms include the greater permeability of tumour vessels than normal vessels to macromolecules and the retention of macromolecules in tumours due to poor lymphatic clearance.
- Computed tomography
-
(CT). A diagnostic imaging test used to create images of internal organs, bones, soft tissue and blood vessels. In oncology, cross-sectional CT images are used to confirm the location and size of tumours.
- Solid stresses
-
Stresses exerted by and accumulated within solid components of tissues (that is, cells and extracellular matrix) during growth and progression. In tumours, solid stress is elevated due to growth and is independent of high interstitial fluid pressure.
- Tumour deformation assays
-
An assay to quantify stress in tumours. Excised tumours are cut in the middle of the tumour, and stress relaxation is quantified as the extent of tumour opening normalized to the diameter of the tumour.
- Desmoplasia
-
The formation and growth of fibrous tissue. In cancer, desmoplasia may occur around a neoplasm, causing dense fibrosis around the tumour.
- Ultrasonography
-
A technique using echoes of ultrasound pulses to delineate objects or areas of different density in the body. In cancer, ultrasonography is used to detect solid tumours.
- Matricellular-enriched fibrosis
-
The thickening and scarring of tissue surrounding a tumour, composed of dynamically expressed, non-structural proteins that are present in the extracellular matrix.
- Hyaluronidase
-
An enzyme that catalyses the degradation of hyaluronic acid, a component of the extracellular matrix that contributes to tumour growth.
- Adjuvant
-
A substance that enhances the body's immune response to foreign antigens.
- Replicon mRNA
-
A self-replicating nucleic acid that amplifies production of the encoded protein and prolongs translation.
- Scavenger receptors
-
A group of receptors that recognize low-density lipoprotein that has been modified by oxidation or acetylation.
- Microdoses
-
Doses of a drug on the microgram scale, or about one-millionth of the systemic dose of a drug, that are intended to produce a beneficial result while avoiding undesirable side effects.
- Bacteriophages
-
Long, tubular viruses that infect specific bacteria; they have been used as scaffolds for nanoparticles and targeting ligands for imaging tumours using magnetic resonance imaging (MRI).
- Autologous chemotaxis
-
A mechanism by which a tumour cell can receive directional cues while at the same time being the source of such cues, enabling dissemination into the lymphatic system.
- Surface plasmon resonance
-
An optical technique for detecting the interaction of two different molecules or particles, in which one is mobile and one is fixed on a thin gold film.
- Deterministic lateral displacement pillar arrays
-
Arrays of pillars fabricated from silicon used to sort, separate and enrich microscale particles including parasites, bacteria, blood cells and tumour cells under flow conditions.
Rights and permissions
About this article
Cite this article
Mitchell, M., Jain, R. & Langer, R. Engineering and physical sciences in oncology: challenges and opportunities. Nat Rev Cancer 17, 659–675 (2017). https://doi.org/10.1038/nrc.2017.83
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc.2017.83
This article is cited by
-
Controlling the biodistribution and clearance of nanomedicines
Nature Reviews Bioengineering (2023)
-
Functional biomaterials for biomimetic 3D in vitro tumor microenvironment modeling
In vitro models (2023)
-
Dose Finding in Oncology: What is Impeding Coming of Age?
Pharmaceutical Research (2022)