Abstract
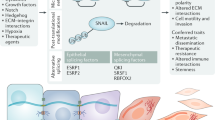
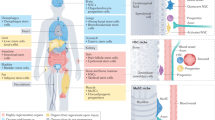
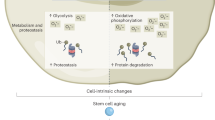
In recent years, great strides have been made in our understanding of how stem cells (SCs) govern tissue homeostasis and regeneration. The inherent longevity of SCs raises the possibility that the unique protective mechanisms in these cells might also be involved in tumorigenesis. In this Opinion article, we discuss how SCs are protected throughout their lifespan, focusing on quiescent behaviour, DNA damage response and programmed cell death. We briefly examine the roles of adult SCs and progenitors in tissue repair and tumorigenesis and explore how signals released from dying or dormant cells influence the function of healthy or aberrant SCs. Important insight into the mechanisms that regulate SC death and survival, as well as the 'legacy' imparted by departing cells, may unlock novel avenues for regenerative medicine and cancer therapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Morrison, S. J. & Spradling, A. C. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell 132, 598–611 (2008).
Mandal, P. K., Blanpain, C. & Rossi, D. J. DNA damage response in adult stem cells: pathways and consequences. Nat. Rev. Mol. Cell Biol. 12, 198–202 (2011).
Nguyen, L. V., Vanner, R., Dirks, P. & Eaves, C. J. Cancer stem cells: an evolving concept. Nat. Rev. Cancer 12, 133–143 (2012).
Blanpain, C. Tracing the cellular origin of cancer. Nat. Cell Biol. 15, 126–134 (2013).
Ge, Y. et al. Stem cell lineage infidelity drives wound repair and cancer. Cell 169, 636–650 (2017).
Youssef, K. K. et al. Identification of the cell lineage at the origin of basal cell carcinoma. Nat. Cell Biol. 12, 299–305 (2010).
Solanas, G. & Benitah, S. A. Regenerating the skin: a task for the heterogeneous stem cell pool and surrounding niche. Nat. Rev. Mol. Cell Biol. 14, 737–748 (2013).
Arwert, E. N., Hoste, E. & Watt, F. M. Epithelial stem cells, wound healing and cancer. Nat. Rev. Cancer 12, 170–180 (2012).
Gurtner, G. C., Werner, S., Barrandon, Y. & Longaker, M. T. Wound repair and regeneration. Nature 453, 314–321 (2008).
Donati, G. & Watt, F. M. Stem cell heterogeneity and plasticity in epithelia. Cell Stem Cell 16, 465–476 (2015).
Blanpain, C. & Fuchs, E. Plasticity of epithelial stem cells in tissue regeneration. Science 344, 1242281 (2014).
Ito, M. et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat. Med. 11, 1351–1354 (2005).
Snippert, H. J. et al. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science 327, 1385–1389 (2010).
Mascré, G. et al. Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature 489, 257–262 (2012).
Aragona, M. et al. Defining stem cell dynamics and migration during wound healing in mouse skin epidermis. Nat. Commun. 8, 14684 (2017).
Park, S. et al. Tissue-scale coordination of cellular behaviour promotes epidermal wound repair in live mice. Nat. Cell Biol. 19, 155–163 (2017).
Ito, M. et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 447, 316–320 (2007).
Chou, W. C. et al. Direct migration of follicular melanocyte stem cells to the epidermis after wounding or UVB irradiation is dependent on Mc1r signaling. Nat. Med. 19, 924–929 (2013).
Yan, K. S. et al. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc. Natl Acad. Sci. USA 109, 466–471 (2012).
Tian, H. et al. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 478, 255–259 (2011).
Sangiorgi, E. & Capecchi, M. R. Bmi1 is expressed in vivo in intestinal stem cells. Nat. Genet. 40, 915–920 (2008).
Barriga, F. M. et al. Mex3a marks a slowly dividing subpopulation of Lgr5+ intestinal stem cells. Cell Stem Cell 20, 801–816 (2017).
Montgomery, R. K. et al. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc. Natl Acad. Sci. USA 108, 179–184 (2011).
Powell, A. E. et al. The pan-ErbB negative regulator lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell 149, 146–158 (2012).
Buczacki, S. J. A. et al. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature 495, 65–69 (2013).
Tetteh, P. W. et al. Replacement of Llost Lgr5-positive stem cells through plasticity of their enterocyte-lineage daughters. Cell Stem Cell 18, 203–213 (2016).
van Es, J. H. et al. Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nat. Cell Biol. 14, 1099–1104 (2012).
Wilson, A. et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell 135, 1118–1129 (2008).
Trumpp, A., Essers, M. & Wilson, A. Awakening dormant haematopoietic stem cells. Nat. Rev. Immunol. 10, 201–209 (2010).
Essers, M. a G. et al. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature 458, 904–908 (2009).
Lumière, A. & Bérard, L. Le Cancer, Maladie des Cicatrices. (Masson, 1929).
Schäfer, M. & Werner, S. Cancer as an overhealing wound: an old hypothesis revisited. Nat. Rev. Mol. Cell Biol. 9, 628–638 (2008).
Dvorak, H. F. Tumors: wounds that do not heal — redux. Cancer Immunol. Res. 3, 1–11 (2015).
Youssef, K. K. et al. Adult interfollicular tumour-initiating cells are reprogrammed into an embryonic hair follicle progenitor-like fate during basal cell carcinoma initiation. Nat. Cell Biol. 14, 1282–1294 (2012).
Wong, S. Y. & Reiter, J. F. Wounding mobilizes hair follicle stem cells to form tumors. Proc. Natl Acad. Sci. USA 108, 4093–4098 (2011).
Wang, G. Y., Wang, J., Mancianti, M. L. & Epstein, E. H. Basal cell carcinomas arise from hair follicle stem cells in Ptch1+/− mice. Cancer Cell 19, 114–124 (2011).
Petersson, M. et al. Interfering with stem cell-specific gatekeeper functions controls tumour initiation and malignant progression of skin tumours. Nat. Commun. 6, 5874 (2015).
Kasper, M. et al. Wounding enhances epidermal tumorigenesis by recruiting hair follicle keratinocytes. Proc. Natl Acad. Sci. USA 108, 4099–4104 (2011).
Sánchez-Danés, A. et al. Defining the clonal dynamics leading to mouse skin tumour initiation. Nature 536, 298–303 (2016).
Malanchi, I. et al. Cutaneous cancer stem cell maintenance is dependent on β-catenin signalling. Nature 452, 650–653 (2008).
White, A. C. et al. Defining the origins of Ras/p53-mediated squamous cell carcinoma. Proc. Natl Acad. Sci. USA 108, 7425–7430 (2011).
Lapouge, G. et al. Identifying the cellular origin of squamous skin tumors. Proc. Natl Acad. Sci. USA 108, 7431–7436 (2011).
Li, S. et al. A keratin 15 containing stem cell population from the hair follicle contributes to squamous papilloma development in the mouse. Mol. Carcinog. 52, 751–759 (2013).
Page, M. E., Lombard, P., Ng, F., Göttgens, B. & Jensen, K. B. The epidermis comprises autonomous compartments maintained by distinct stem cell populations. Cell Stem Cell 13, 471–482 (2013).
Tan, S. & Barker, N. Epithelial stem cells and intestinal cancer. Semin. Cancer Biol. 32, 40–53 (2015).
Vermeulen, L. et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat. Cell Biol. 12, 468–476 (2010).
Zhu, L. et al. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature 457, 603–608 (2009).
Barker, N. et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 457, 608–611 (2009).
Ireland, H., Houghton, C., Howard, L. & Winton, D. J. Cellular inheritance of a Cre-activated reporter gene to determine Paneth cell longevity in the murine small intestine. Dev. Dyn. 233, 1332–1336 (2005).
Melo, F. de S. e et al. A distinct role for Lgr5+ stem cells in primary and metastatic colon cancer. Nature 543, 676–680 (2017).
Asfaha, S. et al. Krt19+/Lgr5− cells are radioresistant cancer-initiating stem cells in the colon and intestine. Cell Stem Cell 16, 627–638 (2015).
Schwitalla, S. et al. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell 152, 25–38 (2013).
Hartnett, L. & Egan, L. J. Inflammation, DNA methylation and colitis-associated cancer. Carcinogenesis 33, 723–731 (2012).
Davidson, L. A. et al. Targeted deletion of p53 in Lgr5-expressing intestinal stem cells promotes colon tumorigenesis in a preclinical model of colitis-associated cancer. Cancer Res. 75, 5392–5397 (2015).
Vitale, I., Manic, G., De Maria, R., Kroemer, G. & Galluzzi, L. DNA damage in stem cells. Mol. Cell 66, 306–319 (2017).
Blanpain, C., Mohrin, M., Sotiropoulou, P. A. & Passegué, E. DNA-damage response in tissue-specific and cancer stem cells. Cell Stem Cell 8, 16–29 (2011).
Sotiropoulou, P. a et al. Bcl-2 and accelerated DNA repair mediates resistance of hair follicle bulge stem cells to DNA-damage-induced cell death. Nat. Cell Biol. 12, 572–582 (2010).
Cheung, T. H. & Rando, T. A. Molecular regulation of stem cell quiescence. Nat. Rev. Mol. Cell Biol. 14, 329–340 (2013).
Li, L. & Clevers, H. Coexistence of quiescent and active adult stem cells in mammals. Science 327, 542–545 (2010).
White, A. C. et al. Stem cell quiescence acts as a tumour suppressor in squamous tumours. Nat. Cell Biol. 16, 99–107 (2014).
Ansell, D. M., Kloepper, J. E., Thomason, H. A., Paus, R. & Hardman, M. J. Exploring the 'hair growth–wound healing connection': anagen phase promotes wound re-epithelialization. J. Invest. Dermatol. 131, 518–528 (2011).
Liu, Y. et al. p53 regulates hematopoietic stem cell quiescence. Cell Stem Cell 4, 37–48 (2009).
Seita, J., Rossi, D. J. & Weissman, I. L. Differential DNA damage response in stem and progenitor cells. Cell Stem Cell 7, 145–147 (2010).
Sperka, T., Wang, J. & Rudolph, K. L. DNA damage checkpoints in stem cells, ageing and cancer. Nat. Rev. Mol. Cell Biol. 13, 579–590 (2012).
Oliver, L. et al. Differentiation-related response to DNA breaks in human mesenchymal stem cells. Stem Cells 31, 800–807 (2013).
Chang, C.-H. et al. Mammary stem cells and tumor-initiating cells are more resistant to apoptosis and exhibit increased DNA repair activity in response to DNA damage. Stem Cell Rep. 5, 378–391 (2015).
Mohrin, M. et al. Hematopoietic stem cell quiescence promotes error-prone DNA repair and mutagenesis. Cell Stem Cell 7, 174–185 (2010).
Wu, W. S. et al. Slug antagonizes p53-mediated apoptosis of hematopoietic progenitors by repressing puma. Cell 123, 641–653 (2005).
Zilfou, J. T., Spector, M. S. & Lowe, S. W. Slugging it out: fine tuning the p53-PUMA death connection. Cell 123, 545–548 (2005).
Beerman, I., Seita, J., Inlay, M. A., Weissman, I. L. & Rossi, D. J. Quiescent hematopoietic stem cells accumulate DNA damage during aging that is repaired upon entry into cell cycle. Cell Stem Cell 15, 37–50 (2014).
Moehrle, B. M. et al. Stem cell-specific mechanisms ensure genomic fidelity within HSCs and upon aging of HSCs. Cell Rep. 13, 2412–2424 (2015).
Rossi, D. J., Jamieson, C. H. M. & Weissman, I. L. Stems cells and the pathways to aging and cancer. Cell 132, 681–696 (2008).
Rossi, D. J. et al. Hematopoietic stem cell quiescence attenuates DNA damage response and permits DNA damage accumulation during aging. Cell Cycle 6, 2371–2376 (2007).
Hua, G. et al. Crypt base columnar stem cells in small intestines of mice are radioresistant. Gastroenterology 143, 1266–1276 (2012).
Barker, N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat. Rev. Mol. Cell Biol. 15, 19–33 (2014).
Barker, N. et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007 (2007).
Potten, C. S. Extreme sensitivity of some intestinal crypt cells to X and gamma irradiation. Nature 269, 518–521 (1977).
Zhu, Y., Huang, Y. F., Kek, C. & Bulavin, D. V. Apoptosis differently affects lineage tracing of lgr5 and bmi1 intestinal stem cell populations. Cell Stem Cell 12, 298–303 (2013).
Ruzankina, Y. et al. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell 1, 113–126 (2007).
Matsumura, H. et al. Hair follicle aging is driven by transepidermal elimination of stem cells via COL17A1 proteolysis. Science 351, aad4395 (2016).
Inomata, K. et al. Genotoxic stress abrogates renewal of melanocyte stem cells by triggering their differentiation. Cell 137, 1088–1099 (2009).
Sotiropoulou, P. a. et al. BRCA1 deficiency in skin epidermis leads to selective loss of hair follicle stem cells and their progeny. Genes Dev. 27, 39–51 (2013).
Nijnik, A. et al. DNA repair is limiting for haematopoietic stem cells during ageing. Nature 447, 686–690 (2007).
White, A. C. & Lowry, W. E. Refining the role for adult stem cells as cancer cells of origin. Trends Cell Biol. 25, 11–20 (2015).
Clevers, H. The cancer stem cell: premises, promises and challenges. Nat. Med. 17, 313–319 (2011).
Saito, Y. et al. Induction of cell cycle entry eliminates human leukemia stem cells in a mouse model of AML. Nat. Biotechnol. 28, 275–280 (2010).
Slupianek, A. et al. Fusion tyrosine kinases induce drug resistance by stimulation of homology-dependent recombination repair, prolongation of G2/M phase, and protection from apoptosis. Mol. Cell. Biol. 22, 4189–4201 (2002).
Slupianek, A., Nowicki, M. O., Koptyra, M. & Skorski, T. BCR/ABL modifies the kinetics and fidelity of DNA double-strand breaks repair in hematopoietic cells. DNA Repair 5, 243–250 (2006).
Nowicki, M. O. et al. BCR/ABL oncogenic kinase promotes unfaithful repair of the reactive oxygen species — dependent DNA double-strand breaks. Am. Soc. Hematol. 104, 3746–3753 (2004).
Perrotti, D., Jamieson, C., Goldman, J. & Skorski, T. Chronic myeloid leukemia: mechanisms of blastic transformation. J. Clin. Invest. 120, 2254–2264 (2010).
Fuchs, Y. & Steller, H. Programmed cell death in animal development and disease. Cell 147, 742–758 (2011).
Fuchs, Y. & Steller, H. Live to die another way: modes of programmed cell death and the signals emanating from dying cells. Nat. Rev. Mol. Cell Biol. 16, 329–344 (2015).
Stenn, K. S., Lawrence, L., Veis, D., Korsmeyer, S. & Seiberg, M. Expression of the bcl-2 protooncogene in the cycling adult mouse hair follicle. J. Invest. Dermatol. 103, 107–111 (1994).
Lindner, G. et al. Analysis of apoptosis during hair follicle regression (catagen). Am. J. Pathol. 151, 1601–1617 (1997).
Nishimura, E. K., Granter, S. R. & Fisher, D. E. Mechanisms of hair graying: incomplete melanocyte stem cell maintenance in the niche. Science 307, 720–724 (2005).
Ito, M., Kizawa, K., Hamada, K. & Cotsarelis, G. Hair follicle stem cells in the lower bulge form the secondary germ, a biochemically distinct but functionally equivalent progenitor cell population, at the termination of catagen. Differentiation 72, 548–557 (2004).
Merritt, A. J. et al. Differential expression of bcl-2 in intestinal epithelia. Correlation with attenuation of apoptosis in colonic crypts and the incidence of colonic neoplasia. J. Cell Sci. 108, 2261–2271 (1995).
van der Heijden, M. et al. Bcl-2 is a critical mediator of intestinal transformation. Nat. Commun. 7, 10916 (2016).
Flohil, C. C., Janssen, P. A. & Bosman, F. T. Expression of Bcl-2 protein in hyperplastic polyps, adenomas, and carcinomas of the colon. J. Pathol. 178, 393–397 (1996).
Motoyama, N. et al. Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science 267, 1506–1510 (1995).
Lagadinou, E. D. et al. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell 12, 329–341 (2013).
Delia, D. et al. bcl-2 proto-oncogene expression in normal and neoplastic human myeloid cells. Blood 79, 1291–1298 (1992).
Campbell, C. J. V. et al. The human stem cell hierarchy is defined by a functional dependence on Mcl-1 for self-renewal capacity. Blood 116, 1433–1442 (2010).
Domen, J. & Weissman, I. L. Hematopoietic stem cells and other hematopoietic cells show broad resistance to chemotherapeutic agents in vivo when overexpressing bcl-2. Exp. Hematol. 31, 631–639 (2003).
Goff, D. J. et al. A Pan-BCL2 inhibitor renders bone-marrow-resident human leukemia stem cells sensitive to tyrosine kinase inhibition. Cell Stem Cell 12, 316–328 (2013).
Campbell, K. J. et al. Elevated Mcl-1 perturbs lymphopoiesis, promotes transformation of hematopoietic stem/progenitor cells, and enhances drug resistance. Blood 116, 3197–3207 (2010).
Larisch, S. et al. A novel mitochondrial septin-like protein, ARTS, mediates apoptosis dependent on its P-loop motif. Nat. Cell Biol. 2, 915–921 (2000).
Elhasid, R. et al. Mitochondrial pro-apoptotic ARTS protein is lost in the majority of acute lymphoblastic leukemia patients. Oncogene 23, 5468–5475 (2004).
Garcia-Fernandez, M. et al. Sept4/ARTS is required forstem cell apoptosis and tumor suppression. Genes Dev. 24, 2282–2293 (2010).
Fuchs, Y. et al. Sept4/ARTS Regulates Stem Cell Apoptosis and Skin Regeneration. Science 341, 286–289 (2013).
Fogarty, C. E. & Bergmann, A. The sound of silence: signaling by apoptotic cells. Curr. Top. Dev. Biol. 114, 241–265 (2015).
Pérez-Garijo, A. & Steller, H. Spreading the word: non-autonomous effects of apoptosis during development, regeneration and disease. Development 142, 3253–3262 (2015).
Chera, S. et al. Apoptotic cells provide an unexpected source of Wnt3 signaling to drive hydra head regeneration. Dev. Cell 17, 279–289 (2009).
Hay, B. a, Wolff, T. & Rubin, G. M. Expression of baculovirus P35 prevents cell death in Drosophila. Development 120, 2121–2129 (1994).
Ryoo, H. D., Gorenc, T. & Steller, H. Apoptotic cells can induce compensatory cell proliferation through the JNK and the wingless signaling pathways. Dev. Cell 7, 491–501 (2004).
Pérez-Garijo, A., Martín, F. a & Morata, G. Caspase inhibition during apoptosis causes abnormal signalling and developmental aberrations in Drosophila. Development 131, 5591–5598 (2004).
Huh, J. R., Guo, M. & Hay, B. A. Compensatory proliferation induced by cell death in the Drosophila wing disc requires activity of the apical cell death caspase dronc in a nonapoptotic role. Curr. Biol. 14, 1262–1266 (2004).
Pérez-Garijo, A., Shlevkov, E. & Morata, G. The role of Dpp and Wg in compensatory proliferation and in the formation of hyperplastic overgrowths caused by apoptotic cells in the Drosophila wing disc. Development 136, 1169–1177 (2009).
Fan, Y. et al. Genetic models of apoptosis-induced proliferation decipher activation of JNK and identify a requirement of EGFR signaling for tissue regenerative responses in Drosophila. PLoS Genet. 10, e1004131 (2014).
Kondo, S., Senoo-Matsuda, N., Hiromi, Y. & Miura, M. DRONC coordinates cell death and compensatory proliferation. Mol. Cell. Biol. 26, 7258–7268 (2006).
Fan, Y. & Bergmann, A. Distinct mechanisms of apoptosis-induced compensatory proliferation in proliferating and differentiating tissues in the Drosophila eye. Dev. Cell 14, 399–410 (2008).
Fogarty, C. E. et al. Extracellular reactive oxygen species drive apoptosis-induced proliferation via Drosophila macrophages. Curr. Biol. 26, 575–584 (2016).
Jiang, H. et al. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell 137, 1343–1355 (2009).
Patel, P. H., Dutta, D. & Edgar, B. A. Niche appropriation by Drosophila intestinal stem cell tumours. Nat. Cell Biol. 17, 1182–1192 (2015).
Bilak, A., Uyetake, L. & Su, T. T. Dying cells protect survivors from radiation-induced cell death in Drosophila. PLoS Genet. 10, e1004220 (2014).
Li, F. et al. Apoptotic cells activate the 'phoenix rising' pathway to promote wound healing and tissue regeneration. Sci. Signal. 3, ra13 (2010).
Goessling, W. et al. Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell 136, 1136–1147 (2009).
Lauber, K. et al. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell 113, 717–730 (2003).
Thomson, J. A., Shapiro, S. S., Waknitz, M. A. & Marshall, V. S. Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 (1998).
Trempus, C. S. et al. Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. J. Invest. Dermatol. 120, 501–511 (2003).
Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 16, 582–598 (2016).
Soteriou, D. et al. Comparative proteomic analysis of supportive and unsupportive extracellular matrix substrates for human embryonic stem cell maintenance. J. Biol. Chem. 288, 18716–18731 (2013).
Huang, Q. et al. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat. Med. 17, 860–866 (2011).
Donato, A. L. et al. Caspase 3 promotes surviving melanoma tumor cell growth after cytotoxic therapy. J. Invest. Dermatol. 134, 1686–1692 (2014).
Liu, X. et al. Caspase-3 promotes genetic instability and carcinogenesis. Mol. Cell 58, 284–296 (2015).
Ichim, G. et al. Limited mitochondrial permeabilization causes DNA damage and genomic instability in the absence of cell death. Mol. Cell 57, 860–872 (2015).
Tang, H. L. et al. Cell survival, DNA damage, and oncogenic transformation after a transient and reversible apoptotic response. Mol. Biol. Cell 23, 2240–2252 (2012).
Cartwright, I. M., Liu, X., Zhou, M., Li, F. & Li, C.-Y. Essential roles of Caspase-3 in facilitating Myc-induced genetic instability and carcinogenesis. eLife 6, e26371 (2017).
Pérez, E., Lindblad, J. L. & Bergmann, A. Tumor-promoting function of apoptotic caspases by an amplification loop involving ROS, macrophages and JNK in Drosophila. eLife 6, e26747 (2017).
Ichim, G. & Tait, S. W. G. A fate worse than death: apoptosis as an oncogenic process. Nat. Rev. Cancer 16, 539–548 (2016).
Visvader, J. E. & Lindeman, G. J. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat. Rev. Cancer 8, 755–768 (2008).
Kurtova, A. V. et al. Blocking PGE2-induced tumour repopulaiton abrogates bladder cancer chemoresistance. Nature 517, 209–213 (2014).
Chammas, R., de Sousa Andrade, L. N. & Jancar, S. Oncogenic effects of PAFR ligands produced in tumours upon chemotherapy and radiotherapy. Nat. Rev. Cancer 17, 253–253 (2017).
Mao, P., Smith, L., Xie, W. & Wang, M. Dying endothelial cells stimulate proliferation of malignant glioma cells via a caspase 3-mediated pathway. Oncol. Lett. 5, 1615–1620 (2013).
Morata, G. & Martín, F. A. Cell competition: the embrace of death. Dev. Cell 13, 1–2 (2007).
Moreno, E. Is cell competition relevant to cancer? Nat. Rev. Cancer 8, 141–147 (2008).
Suijkerbuijk, S. J. E., Kolahgar, G., Kucinski, I. & Piddini, E. Cell competition drives the growth of intestinal adenomas in Drosophila. Curr. Biol. 26, 428–438 (2016).
Eichenlaub, T., Cohen, S. M. & Herranz, H. Cell competition drives the formation of metastatic tumors in a drosophila model of epithelial tumor formation. Curr. Biol. 26, 419–427 (2016).
Ballesteros-Arias, L., Saavedra, V. & Morata, G. Cell competition may function either as tumour-suppressing or as tumour-stimulating factor in Drosophila. Oncogene 33, 4377–4384 (2014).
Kolahgar, G. et al. Cell competition modifies adult stem cell and tissue population dynamics in a JAK-STAT-dependent manner. Dev. Cell 34, 297–309 (2015).
Bondar, T. & Medzhitov, R. p53-mediated hematopoietic stem and progenitor cell competition. Cell Stem Cell 6, 309–322 (2010).
Dumble, M. et al. The impact of altered p53 dosage on hematopoietic stem cell dynamics during aging. Blood 109, 1736–1742 (2007).
Stine, R. R. & Matunis, E. L. Stem cell competition: finding balance in the niche. Trends Cell Biol. 23, 357–364 (2013).
Sancho, M. et al. Competitive interactions eliminate unfit embryonic stem cells at the onset of differentiation. Dev. Cell 26, 19–30 (2013).
Clavería, C., Giovinazzo, G., Sierra, R. & Torres, M. Myc-driven endogenous cell competition in the early mammalian embryo. Nature 500, 39–44 (2013).
Brown, S. et al. Correction of aberrant growth preserves tissue homeostasis. Nature 548, 334–337 (2017).
Pérez-Garijo, A., Fuchs, Y. & Steller, H. Apoptotic cells can induce non-autonomous apoptosis through the TNF pathway. eLife 2, e01004 (2013).
Prise, K. M. & O'Sullivan, J. M. Radiation-induced bystander signalling in cancer therapy. Nat. Rev. Cancer 9, 351–360 (2009).
Gauron, C. et al. Sustained production of ROS triggers compensatory proliferation and is required for regeneration to proceed. Sci. Rep. 3, 2084 (2013).
Nguyen-Chi, M. et al. TNF signaling and macrophages govern fin regeneration in zebrafish larvae. Cell Death Dis. 8, e2979 (2017).
Bhola, P. D. & Letai, A. Mitochondria-judges and executioners of cell death sentences. Mol. Cell 61, 695–704 (2016).
Delbridge, A. R. D., Grabow, S., Strasser, A. & Vaux, D. L. Thirty years of BCL-2: translating cell death discoveries into novel cancer therapies. Nat. Rev. Cancer 16, 99–109 (2016).
Fulda, S. & Vucic, D. Targeting IAP proteins for therapeutic intervention in cancer. Nat. Rev. Drug Discov. 11, 109–124 (2012).
Ashkenazi, A., Fairbrother, W. J., Leverson, J. D. & Souers, A. J. From basic apoptosis discoveries to advanced selective BCL-2 family inhibitors. Nat. Rev. Drug Discov. 16, 273–284 (2017).
Chonghaile, T. N. et al. Pretreatment mitochondrial priming correlates with clinical response to cytotoxic chemotherapy. Science 334, 1129–1133 (2011).
Sarosiek, K. A. et al. Developmental regulation of mitochondrial apoptosis by c-Myc governs age- and tissue-specific sensitivity to cancer therapeutics. Cancer Cell 31, 142–156 (2017).
Certo, M. et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell 9, 351–365 (2006).
Vo, T. T. et al. Relative mitochondrial priming of myeloblasts and normal HSCs determines chemotherapeutic success in AML. Cell 151, 344–355 (2012).
Koren, E. & Fuchs, Y. The bad seed: cancer stem cells in tumor development and resistance. Drug Resist. Updat. 28, 1–12 (2016).
Carter, B. Z. et al. Combined targeting of BCL-2 and BCR-ABL tyrosine kinase eradicates chronic myeloid leukemia stem cells. Sci. Transl Med. 8, 355ra117 (2016).
Carter, B. Z. et al. Synergistic targeting of AML stem/progenitor cells with IAP antagonist birinapant and demethylating agents. J. Natl. Cancer Inst. 106, djt440 (2014).
Carter, B. Z. et al. XIAP antisense oligonucleotide (AEG35156) achieves target knockdown and induces apoptosis preferentially in CD34+38- cells in a phase 1/2 study of patients with relapsed/refractory AML. Apoptosis 16, 67–74 (2011).
Pérez-Mancera, P. A., Young, A. R. J. & Narita, M. Inside and out: the activities of senescence in cancer. Nat. Rev. Cancer 14, 547–558 (2014).
Muñoz-Espín, D. & Serrano, M. Cellular senescence: from physiology to pathology. Nat. Rev. Mol. Cell Biol. 15, 482–496 (2014).
Chang, J. et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 22, 78–83 (2016).
Campisi, J. & d'Adda di Fagagna, F. Cellular senescence: when bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 8, 729–740 (2007).
de Keizer, P. L. J. The fountain of youth by targeting senescent cells? Trends Mol. Med. 23, 6–17 (2017).
Serrano, M. Senescence helps regeneration. Dev. Cell 31, 671–672 (2014).
Demaria, M. et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell 31, 722–733 (2014).
Ritschka, B. et al. The senescence-associated secretory phenotype induces cellular plasticity and tissue regeneration. Genes Dev. 31, 172–183 (2017).
Mosteiro, L. et al. Tissue damage and senescence provide critical signals for cellular reprogramming in vivo. Science 354, aaf4445 (2016).
Chiche, A. et al. Injury-induced senescence enables in vivo reprogramming in skeletal muscle. Cell Stem Cell 20, 407–414 (2017).
Krtolica, A., Parrinello, S., Lockett, S., Desprez, P.-Y. & Campisi, J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc. Natl Acad. Sci. USA 98, 12072–12077 (2001).
Yoshimoto, S. et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 499, 97–101 (2013).
Merritt, A. J. et al. The role of p53 in spontaneous and radiation-induced apoptosis in the gastrointestinal tract of normal and p53-deficient mice. Cancer Res. 54, 614–617 (1994).
Chang, H. H. Y., Pannunzio, N. R., Adachi, N. & Lieber, M. R. Non-homologous DNA end joining and alternative pathways to double-strand break repair. Nat. Rev. Mol. Cell Biol. 18, 495–506 (2017).
Panier, S. & Boulton, S. J. Double-strand break repair: 53BP1 comes into focus. Nat. Rev. Mol. Cell Biol. 15, 7–18 (2013).
Tait, S. W. G. & Green, D. R. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat. Rev. Mol. Cell Biol. 11, 621–632 (2010).
Salvesen, G. S. & Walsh, C. M. Functions of caspase 8: the identified and the mysterious. Semin. Immunol. 26, 246–252 (2014).
Pasparakis, M. & Vandenabeele, P. Necroptosis and its role in inflammation. Nature 517, 311–320 (2015).
Liu, J. C., Lerou, P. H. & Lahav, G. Stem cells: balancing resistance and sensitivity to DNA damage. Trends Cell Biol. 24, 268–274 (2014).
Martinou, J. C. & Youle, R. J. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev. Cell 21, 92–101 (2011).
Salvesen, G. S. & Duckett, C. S. IAP proteins: blocking the road to death's door. Nat. Rev. Mol. Cell Biol. 3, 401–410 (2002).
Bratton, S. B. & Salvesen, G. S. Regulation of the Apaf-1-caspase-9 apoptosome. J. Cell Sci. 123, 3209–3214 (2010).
Acknowledgements
The authors apologize to colleagues whose contributions could not be adequately cited because of space constraints. The authors thank E. Koren, I. Maniv, Y. Yosefzon and A. Pérez-Garijo for discussion, advice and assistance. D.S. is supported by the Aly-Kaufman and Coleman-Cohen fellowship. Y.F. is the Deloro Career Advancement Chair and is supported by the German-Israeli Foundation for Scientific Research and Development (GIF; I-2381-412.13/2015) and Israel Cancer Research Fund (ICRF; 15-771-RCDA) grants.
Author information
Authors and Affiliations
Contributions
Both authors researched data for the article; made substantial contributions to discussing the content; and wrote, reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Adult stem cells
-
(SCs). Stem cells found in different tissues in the adult organism that divide to replenish tissues and organs.
- Alopecia
-
Loss of hair from parts of the head or body.
- BH3 mimetics
-
A class of small-molecule compounds that mimic the action of proapoptotic BH3-only proteins by binding the prosurvival BCL-2 family members and consequently activating apoptosis in cells that express these proteins.
- BH3 profiling
-
A tool used to predict the cellular response to different synthetic BH3 peptides that mimic BH3-only proteins. It provides information on which BCL-2 family protein is required for survival of specific cell types (healthy or malignant).
- Blastema
-
A transient structure formed during regeneration that consists of a proliferating mass of a morphologically homogeneous population of undifferentiated cells.
- Cancer stem cell
-
(CSC). An immortal cell that resides within the tumour and is equipped with the capacity to self-renew and differentiate into different cell types that make up the tumour.
- Caspases
-
A family of cysteine proteases that cleave various substrates in the cell to implement apoptosis.
- Homologous recombination
-
(HR). A DNA double strand break repair mechanism in which the genomic sequence of the broken DNA ends is restored by using sister chromatids as a template for the repair.
- Interfollicular epidermis
-
(IFE). A stratified squamous epithelium consisting of a basal layer of proliferative cells that differentiate while they migrate upwards to form the outermost layers of the skin.
- Keratin pearls
-
A keratinized structure formed by concentric layers of malignant squamous cells that reside in the centre of most squamous cell carcinomas.
- Lineage tracing
-
The process of identifying all progeny of a single cell.
- Nonhomologous end joining
-
(NHEJ). A DNA double strand break repair mechanism in which damaged DNA is repaired by bringing together the two broken ends and rejoining them by DNA ligation, resulting in small loss of nucleotides.
- Progenitor cells
-
Dividing cells with the capacity to differentiate into a restricted lineage.
- Quiescent
-
A reversible state of cell cycle arrest where cells cease to proliferate but retain their ability to re-enter the cell cycle upon specific signals.
- Self-renewal
-
The process by which stem cells generate additional stem cells to maintain the stem cell pool.
- Senescence
-
An irreversible state of cell cycle arrest characterized by sustained metabolic activity, changes in morphology and unresponsiveness to growth factors.
- Stem cell niches
-
The microenvironments where stem cells reside, which maintain and regulate their fate.
Rights and permissions
About this article
Cite this article
Soteriou, D., Fuchs, Y. A matter of life and death: stem cell survival in tissue regeneration and tumour formation. Nat Rev Cancer 18, 187–201 (2018). https://doi.org/10.1038/nrc.2017.122
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc.2017.122
This article is cited by
-
Bioenergetic profile and redox tone modulate in vitro osteogenesis of human dental pulp stem cells: new perspectives for bone regeneration and repair
Stem Cell Research & Therapy (2023)
-
To not love thy neighbor: mechanisms of cell competition in stem cells and beyond
Cell Death & Differentiation (2023)
-
Targeting LGSN restores sensitivity to chemotherapy in gastric cancer stem cells by triggering pyroptosis
Cell Death & Disease (2023)
-
The role of the immune microenvironment in bone, cartilage, and soft tissue regeneration: from mechanism to therapeutic opportunity
Military Medical Research (2022)
-
Apoptotic stress-induced FGF signalling promotes non-cell autonomous resistance to cell death
Nature Communications (2021)