Key Points
-
Metastases, and complications of their treatment, are significant causes of patient morbidity and mortality. Drugging metastasis pathways represents a potential new therapeutic opportunity.
-
Most preclinical experiments demonstrate partial prevention of metastasis rather than shrinkage of existing lesions. These data would apply to the prevention of an initial metastasis in a high-risk patient, or the prevention of additional metastases in patients with treated, limited metastatic disease.
-
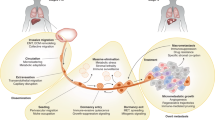
Metastatic colonization represents the best 'open' therapeutic window in metastasis. It is the progressive outgrowth of tumour cells in a distant location, influenced by tumour cell signalling and interaction with a modified microenvironment (the formation of a premetastatic niche, alterations in the extracellular matrix and stromal cells, innate and T cell immunity and altered vascular supply).
-
Denosumab, a monoclonal antibody that blocks the receptor activator of NF-κB ligand (RANKL; which is involved in the bone metastatic process), provides evidence that metastasis can be successfully drugged. Denosumab clinical trials used an interesting primary end point — skeletal-related events (SREs).
-
The development of metastasis prevention agents may be hindered by their cytostatic nature, which will not result in shrinkage of established metastatic lesions (responses) in early clinical testing. New clinical trial designs for primary and secondary metastasis prevention are needed to lower the time, cost and cohort sizes of traditional adjuvant trials.
-
It is likely that a single metastasis-preventive agent will not be maximally effective. As for HIV, a combination of distinct classes of drugs, given early and continuously, will be key.
Abstract
Tumour metastasis, the movement of tumour cells from a primary site to progressively colonize distant organs, is a major contributor to the deaths of cancer patients. Therapeutic goals are the prevention of an initial metastasis in high-risk patients, shrinkage of established lesions and prevention of additional metastases in patients with limited disease. Instead of being autonomous, tumour cells engage in bidirectional interactions with metastatic microenvironments to alter antitumour immunity, the extracellular milieu, genomic stability, survival signalling, chemotherapeutic resistance and proliferative cycles. Can targeting of these interactions significantly improve patient outcomes? In this Review preclinical research, combination therapies and clinical trial designs are re-examined.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Brosnan, J. A. & Iacobuzio-Donahue, C. A. A new branch on the tree: next-generation sequencing in the study of cancer evolution. Semin. Cell Dev. Biol. 23, 237–242 (2012).
Paget, S. The distribution of secondary growths in cancer of the breast. Lancet 1, 99–101 (1889). The origin of the seed and soil hypothesis of metastasis.
Hensel, J. A., Flaig, T. W. & Theodorescu, D. Clinical opportunities and challenges in targeting tumour dormancy. Nat. Rev. Clin. Oncol. 10, 41–51 (2013).
Husemann, Y. et al. Systemic spread is an early step in breast cancer. Cancer Cell 13, 58–68 (2008).
Bojovic, B. & Crowe, D. L. Dysfunctional telomeres promote genomic instability and metastasis in the absence of telomerase activity in oncogene induced mammary cancer. Mol. Carcinogen. 52, 103–117 (2013).
Vermaat, J. S. et al. Primary colorectal cancers and their subsequent hepatic metastases are genetically different: implications for selection of patients for targeted treatment. Clin. Cancer Res. 18, 688–699 (2012).
Yachida, S. et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 467, 1114–1126 (2010).
Roschke, A. et al. Chromosomal instability is associated with higher expression of genes implicated in epithelial-mesenchymal transition, cancer invasiveness, and metastasis and with lower expression of genes involved in cell cycle checkpoints, DNA repair, and chromatin maintenance. Neoplasia 10, 1222–1230 (2008).
Hong, M. K. H. et al. Tracking the origins and drivers of subclonal metastatic expansion in prostate cancer. Nat. Commun. 6, 6605 (2015). This paper showed that prostate cancer metastases harbour actionable mutations not found in matched primary tumours.
Luzzi, K. J. et al. Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am. J. Pathol. 153, 865–873 (1998).
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2015. CA Cancer J. Clin. 65, 5–29 (2015).
Jemal, A. et al. Cancer statistics, 2005. CA Cancer J. Clin. 55, 10–30 (2005).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010). This paper reported the improved OS of patients with metastatic melanoma using an immune checkpoint inhibitor.
Scher, H. I. et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 367, 1187–1197 (2012).
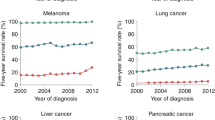
Tevaarwerk, A. J. et al. Survival in patients with metastatic recurrent breast cancer after adjuvant chemotherapy: little evidence of improvement over the past 30 years. Cancer 119, 1140–1148 (2013).
Bernards, N. et al. No improvement in median survival for patients with metastatic gastric cancer despite increased use of chemotherapy. Ann. Oncol. 24, 3056–3060 (2013).
Worni, M. et al. Modest improvement in overall survival for patients with metastatic pancreatic cancer: a trend analysis using the Surveillance, Epidemiology, and End Results registry from 1988 to 2008. Pancreas 42, 1157–1163 (2013).
Lin, T. H. et al. Differential androgen deprivation therapies with anti-androgens casodex/bicalutamide or MDV3100/enzalutamide versus anti-androgen receptor ASC-J9 (R) lead to promotion versus suppression of prostate cancer metastasis. J. Biol. Chem. 288, 19359–19369 (2013).
Sanchez-Laorden, B. et al. BRAF inhibitors induce metastasis in RAS mutant or inhibitor-resistant melanoma cells by reactivating MEK and ERK signaling. Sci. Signal. 7, ra30 (2014).
Volk-Draper, L. et al. Paclitaxel therapy promotes breast cancer metastasis in a TLR4-dependent manner. Cancer Res. 74, 5421–5434 (2014).
Gunjal, P. M. et al. Evidence for induction of a tumor metastasis-receptive microenvironment for ovarian cancer cells in bone marrow and other organs as an unwanted and underestimated side effect of chemotherapy/radiotherapy. J. Ovarian Res. 8, 20 (2015).
Lin, T. H. et al. Anti-androgen receptor ASC-J9 versus anti-androgens MDV3100 (enzalutamide) or casodex (bicalutamide) leads to opposite effects on prostate cancer metastasis via differential modulation of macrophage infiltration and STAT3-CCL2 signaling. Cell Death Dis. 4, e764 (2013).
Pool, S. E. et al. mTOR Inhibitor RAD001 promotes metastasis in a rat model of pancreatic neuroendocrine cancer. Cancer Res. 73, 12–18 (2013).
Guerin, E., Man, S., Xu, P. & Kerbel, R. S. A. Model of postsurgical advanced metastatic breast cancer more accurately replicates the clinical efficacy of antiangiogenic drugs. Cancer Res. 73, 2743–2748 (2013).
Ratajczak, M. Z., Jadczyk, T., Schneider, G., Kakar, S. S. & Kucia, M. Induction of a tumor-metastasis-receptive microenvironment as an unwanted and underestimated side effect of treatment by chemotherapy or radiotherapy. J. Ovarian Res. 6, 95 (2013).
McLeskey, S. W. et al. Fibroblast growth factor-IV transfection of MCF-7 cells produces cell-lines that are tumorigenic and metastatic in ovariectomized or tamoxifen-treated athymic nude-mice. Cancer Res. 53, 2168–2177 (1993).
Onoda, J. M., Jacobs, J. R., Taylor, J. D., Sloane, B. F. & Honn, K. V. Cisplatin and nifedipine: synergistic cytotoxicity against murine solid tumors and their metastases. Cancer Lett. 30, 181–188 (1986).
Mundy, G. Metastasis to the bone: causes, consequences and therapeutic opportunities. Nat. Rev. Cancer 2, 584–593 (2002).
Kostenuik, P. J. et al. Denosumab, a fully human monoclonal antibody to RANKL, inhibits bone resorption and increases BMD in knock-in mice that express chimeric (murine/human) RANKL. J. Bone Miner. Res. 24, 182–195 (2009).
Fizazi, K. et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet 377, 813–822 (2011).
Stopeck, A. T. et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J. Clin. Oncol. 28, 5132–5139 (2010). Phase III trial of denosumab to improve the time until an SRE in bone metastatic breast cancer.
Smith, M. R. et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet 379, 39–46 (2012). Phase III trial demonstrating improvement of bone metastasis-free survival in prostate cancer.
Gnant, M. et al. Adjuvant denosumab in breast cancer (ABCSG-18): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 386, 433–443 (2015).
Folkman, J. Role of angiogenesis in tumor growth and metastasis. Semin. Oncol. 29, 15–18 (2002).
Aghajanian, C. et al. Final overall survival and safety analysis of OCEANS, a phase 3 trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent ovarian cancer. Gynecol. Oncol. 139, 10–16 (2015).
Perren, T. J. et al. A phase 3 trial of bevacizumab in ovarian cancer. N. Engl. J. Med. 365, 2484–2496 (2011).
Herbst, R. S. et al. Efficacy of bevacizumab plus erlotinib versus erlotinib alone in advanced non-small-cell lung cancer after failure of standard first-line chemotherapy (BeTa): a double-blind, placebo-controlled, phase 3 trial. Lancet 377, 1846–1854 (2011).
Sledge, G. W. Anti-vascular endothelial growth factor therapy in breast cancer: game over? J. Clin. Oncol. 33, 133–135 (2015).
Kim, K. B. et al. BEAM: a randomized phase II study evaluating the activity of bevacizumab in combination with carboplatin plus paclitaxel in patients with previously untreated advanced melanoma. J. Clin. Oncol. 30, 34–41 (2012).
Kindler, H. L. et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J. Clin. Oncol. 28, 3617–3622 (2010).
Cameron, D. et al. Adjuvant bevacizumab-containing therapy in triple-negative breast cancer (BEATRICE): primary results of a randomised, phase 3 trial. Lancet Oncol. 14, 933–942 (2013).
de Gramont, A. et al. Bevacizumab plus oxaliplatin-based chemotherapy as adjuvant treatment for colon cancer (AVANT): a phase 3 randomised controlled trial. Lancet Oncol. 13, 1225–1233 (2012).
Allegra, C. J. et al. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP protocol C-08. J. Clin. Oncol. 29, 11–16 (2011).
von Minckwitz, G. et al. Neoadjuvant chemotherapy and bevacizumab for HER2-negative breast cancer. N. Engl. J. Med. 366, 299–309 (2012).
Bear, H. D. et al. Bevacizumab added to neoadjuvant chemotherapy for breast cancer. N. Engl. J. Med. 366, 310–320 (2012).
Weisshardt, P. et al. Tumor vessel stabilization and remodeling by anti-angiogenic therapy with bevacizumab. Histochem. Cell Biol. 137, 391–401 (2012).
Fan, F. et al. Chronic exposure of colorectal cancer cells to bevacizumab promotes compensatory pathways that mediate tumour cell migration. Br. J. Cancer 104, 1270–1277 (2011).
De Groot, J. F. et al. Tumor invasion after treatment of glioblastoma with bevacizumab: radiographic and pathologic correlation in humans and mice. Neuro-Oncol. 12, 233–242 (2010).
Yin, T. et al. Antiangiogenic therapy using sunitinib combined with rapamycin retards tumor growth but promotes metastasis. Transl Oncol. 7, 221–229 (2014).
Ebos, J. et al. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell 15, 232–239 (2009).
Paez-Ribes, M. et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell 15, 220–231 (2009). This paper reports that VEGF inhibitors inhibited tumour growth but accelerated progression in mouse model systems.
Mazzieri, R. et al. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell 19, 512–526 (2011).
Vermeulen, P. B. et al. Liver metastases from colorectal adenocarcinomas grow in three patterns with different angiogenesis and desmoplasia. J. Pathol. 195, 336–342 (2001).
Stessels, F. et al. Breast adenocarcinoma liver metastases, in contrast to colorectal cancer liver metastases, display a non-angiogenic growth pattern that preserves the stroma and lacks hypoxia. Br. J. Cancer 90, 1429–1436 (2004).
Kienast, Y. et al. Real-time imaging reveals the single steps of brain metastasis formation. Nat. Med. 16, 116–122 (2010).
Kusters, B. et al. Differential effects of vascular endothelial growth factor A isoforms in a mouse brain metastasis model of human melanoma. Cancer Res. 63, 5408–5413 (2003).
Maniotis, A. et al. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am. J. Pathol. 155, 739–752 (1999).
Sottnik, J. L. et al. Integrin α2β1 (α2β1) promotes prostate cancer skeletal metastasis. Clin. Exp. Metastasis 30, 569–578 (2013).
Zhou, B. et al. Integrin α3 β1 can function to promote spontaneous metastasis and lung colonization of invasive breast carcinoma. Mol. Cancer Res. 12, 143–154 (2014).
Shibue, T., Brooks, M. W. & Weinberg, R. A. An integrin-linked machinery of cytoskeletal regulation that enables experimental tumor initiation and metastatic colonization. Cancer Cell 24, 481–498 (2013).
Oku, N. et al. Liposomal ARG-GLY-ASP analogs effectively inhibit metastatic B16 melanoma colonization in murine lungs. Life Sci. 58, 2263–2270 (1996).
Hardan, I. et al. Inhibition of metastatic cell colonization in murine lungs and tumor-induced morbidity by nonpeptidic Arg-Gly-Asp mimetics. Int. J. Cancer 55, 1023–1028 (1993).
Tentori, L. et al. The integrin antagonist cilengitide increases the antitumor activity of temozolomide against malignant melanoma. Oncol. Rep. 19, 1039–1043 (2008).
Yamada, S. et al. Effect of the angiogenesis inhibitor cilengitide on glioblastoma growth in nude mice. Neurosurgery 59, 1304–1312 (2006).
Mason, W. P. End of the road: confounding results of the CORE trial terminate the arduous journey of cilengitide for glioblastoma. Neuro-Oncol. 17, 634–635 (2015).
Manegold, C. et al. Randomized phase II study of three doses of the integrin inhibitor cilengitide versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer. Investigat. New Drugs 31, 175–182 (2013).
Alva, A. et al. Phase II study of cilengitide (EMD 121974, NSC 707544) in patients with non-metastatic castration resistant prostate cancer, NCI-6735. A study by the DOD/PCF prostate cancer clinical trials consortium. Investigat. New Drugs 30, 749–757 (2012).
Kim, K. B. et al. A randomized phase II study of cilengitide in patients with metastatic melanoma. Melanoma Res. 22, 294–301 (2012).
Kim, L. C., Song, L. X. & Haura, E. B. Src kinases as therapeutic targets for cancer. Nat. Rev. Clin. Oncol. 6, 587–595 (2009).
Yori, J. L. et al. Combined SFK/mTOR inhibition prevents rapamycin-induced feedback activation of AKT and elicits efficient tumor regression. Cancer Res. 74, 4762–4771 (2014).
Gucalp, A. et al. Phase II trial of saracatinib (AZD0530), an oral SRC-inhibitor for the treatment of patients with hormone receptor-negative metastatic breast cancer. Clin. Breast Cancer 11, 306–311 (2011).
Finn, R. S. et al. Dasatinib as a single agent in triple-negative breast cancer: results of an open-label phase 2 study. Clin. Cancer Res. 17, 6905–6913 (2011).
Mayer, E. L. et al. A phase 2 trial of dasatinib in patients with advanced HER2-positive and/or hormone receptor-positive breast cancer. Clin. Cancer Res. 17, 6897–6904 (2011).
Schilder, R. J. et al. Phase II evaluation of dasatinib in the treatment of recurrent or persistent epithelial ovarian or primary peritoneal carcinoma: a Gynecologic Oncology Group study. Gynecol. Oncol. 127, 70–74 (2012).
Sharma, M. R. et al. Dasatinib in previously treated metastatic colorectal cancer: a phase II trial of the University of Chicago Phase II Consortium. Investigat. New Drugs 30, 1211–1215 (2012).
Kluger, H. M. et al. A Phase 2 trial of dasatinib in advanced melanoma. Cancer 117, 2202–2208 (2011).
Gangadhar, T. C., Clark, J. I., Karrison, T. & Gajewski, T. F. Phase II study of the Src kinase inhibitor saracatinib (AZD0530) in metastatic melanoma. Investigat. New Drugs 31, 769–773 (2013).
Molina, J. R. et al. A phase II trial of the Src-kinase inhibitor saracatinib after four cycles of chemotherapy for patients with extensive stage small cell lung cancer: NCCTG trial N-0621. Lung Cancer 85, 245–250 (2014).
Fury, M. G. et al. Phase II study of saracatinib (AZD0530) for patients with recurrent or metastatic head and neck squamous cell carcinoma (HNSCC). Anticancer Res. 31, 249–253 (2011).
Mackay, H. J. et al. A phase II trial of the Src kinase inhibitor saracatinib (AZD0530) in patients with metastatic or locally advanced gastric or gastro-esophageal junction (GEJ) adenocarcinoma: a trial of the PMH phase II consortium. Investigat. New Drugs 30, 1158–1163 (2012).
Pusztai, L. et al. Gene signature-guided dasatinib therapy in metastatic breast cancer. Clin. Cancer Res. 20, 5265–5271 (2014).
Yang, J. C. et al. Effect of the specific Src family kinase inhibitor saracatinib on osteolytic lesions using the PC-3 bone model. Mol. Cancer Ther. 9, 1629–1637 (2010).
Koreckij, T. et al. Dasatinib inhibits the growth of prostate cancer in bone and provides additional protection from osteolysis. Br. J. Cancer 101, 263–268 (2009).
Twardowski, P. W. et al. A phase II trial of dasatinib in patients with metastatic castration-resistant prostate cancer treated previously with chemotherapy. Anti-Cancer Drugs 24, 743–753 (2013).
Yu, E. Y. et al. Once-daily dasatinib: expansion of phase II study evaluating safety and efficacy of dasatinib in patients with metastatic castration-resistant prostate cancer. Urology 77, 1166–1171 (2011).
Yu, E. Y. et al. Phase II study of dasatinib in patients with metastatic castration-resistant prostate cancer. Clin. Cancer Res. 15, 7421–7428 (2009).
Araujo, J. C. et al. Docetaxel and dasatinib or placebo in men with metastatic castration-resistant prostate cancer (READY): a randomised, double-blind phase 3 trial. Lancet Oncol. 14, 1307–1316 (2013).
Gerlinger, M. et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 366, 883–892 (2012).
Braun, S. et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N. Engl. J. Med. 353, 793–802 (2005).
Schumacher, T. & Schreiber, R. Neoantigens in cancer immunotherapy. Science 348, 69–74 (2015). This study undertook neoantigen quantification in multiple cancer types and demonstrated its potential relevance to immunotherapy.
Fidler, I. Critical factors in the biology of human cancer metastasis. Am. Surg. 61, 1065–1066 (1995).
Taichman, R. S. et al. GAS6 receptor status is associated with dormancy and bone metastatic tumor formation. PLoS ONE 8, e61873 (2013).
Lawson, M. A. et al. Osteoclasts control reactivation of dormant myeloma cells by remodelling the endosteal niche. Nat. Commun. 6, 9983 (2015).
Aguirre-Ghiso, J. A., Estrada, Y., Liu, D. & Ossowski, L. ERKMAPK activity as a determinant of tumor growth and dormancy: regulation by p38(SAPK). Cancer Res. 63, 1684–1695 (2003).
Marshall, J. C. A. et al. Effect of inhibition of the lysophosphatidic acid receptor 1 on metastasis and metastatic dormancy in breast cancer. J. Natl Cancer Inst. 104, 1306–1319 (2012). An LPAR1 inhibitor prevented metastasis and induced aspects of metastatic dormancy.
Lawson, D. A. et al. Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells. Nature 526, 131–135 (2015).
Lu, X. et al. VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging α4β1-positive osteoclast progenitors. Cancer Cell 20, 701–714 (2011).
Barkan, D. et al. Inhibition of metastatic outgrowth from single dormant tumor cells by targeting the cytoskeleton. Cancer Res. 68, 6241–6250 (2008).
Naumov, G. et al. Ineffectiveness of doxorubicin treatment on solitary dormant mammary carcinoma cells or late developing metastases. Breast Cancer Res. Treat. 82, 199–206 (2003). Metastases from an aggressive and a dormant cell line responded differently to chemotherapy.
Goss, P. E. & Chambers, A. F. Does tumour dormancy offer a therapeutic target? Nat. Rev. Cancer 10, 871–877 (2010).
Albanese, I. et al. Heterogeneity within and between primary colorectal carcinomas and matched metastases as revealed by analysis of Ki-ras and p53 mutations. Biochem. Biophys. Res. Commun. 325, 784–791 (2004).
Bartosch, C. et al. Endometrial endometrioid carcinoma metastases show decreased ER-α and PR-A expression compared to matched primary tumors. PLoS ONE 10, e0134969 (2015).
Jordan, V. C., Curpan, R. & Maximov, P. Y. Estrogen receptor mutations found in breast cancer metastases integrated with the molecular pharmacology of selective ER modulators. J. Natl Cancer Inst. 107, djv075 (2015).
Singhi, A. D. et al. MYC gene amplification is often acquired in lethal distant breast cancer metastases of unamplified primary tumors. Modern Pathol. 25, 378–387 (2012).
Colombino, M. et al. BRAF/NRAS mutation frequencies among primary tumors and metastases in patients with melanoma. J. Clin. Oncol. 30, 2522–2529 (2012).
Eriksson, H. et al. BRAF(V600E) protein expression in primary cutaneous malignant melanomas and paired metastases. JAMA Dermatol. 151, 410–416 (2015).
Surriga, O. et al. Crizotinib, a c-Met inhibitor, prevents metastasis in a metastatic uveal melanoma model. Mol. Cancer Ther. 12, 2817–2826 (2013).
Moody, S. E. et al. Conditional activation of Neu in the mammary epithelium of transgenic mice results in reversible pulmonary metastasis. Cancer Cell 2, 451–461 (2002).
Lenfert, E. et al. Mutant p53 promotes epithelial-mesenchymal plasticity and enhances metastasis in mammary carcinomas of WAP-T mice. Int. J. Cancer 136, E521–E533 (2015).
Morton, J. P. et al. Mutant p53 drives metastasis and overcomes growth arrest/senescence in pancreatic cancer. Proc. Natl Acad. Sci. USA 107, 246–251 (2010).
Bandyopadhyay, A., Wang, L., Chin, S. H. & Sun, L. Z. Inhibition of skeletal metastasis by ectopic ERα expression in ERα-negative human breast cancer cell lines. Neoplasia 9, 113–118 (2007).
Brastianos, P. et al. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov. 5, 1164–1177 (2015). Fifty-three per cent of brain metastases harboured actionable mutations not detected in the matched primary tumour.
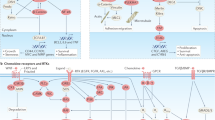
Amersi, F. F. et al. Activation of CCR9/CCL25 in cutaneous melanoma mediates preferential metastasis to the small intestine. Clin. Cancer Res. 14, 638–645 (2008).
Kitamura, T. et al. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J. Exp. Med. 212, 1043–1059 (2015).
Zheng, J. et al. Chemokine receptor CX3CR1 contributes to macrophage survival in tumor metastasis. Mol. Cancer 12, 141 (2013).
Biragyn, A. et al. Inhibition of lung metastasis by chemokine CCL17-mediated in vivo silencing of genes in CCR4+ Tregs. J. Immunother. 36, 258–267 (2013).
Kee, J. Y. et al. Chemokine CXCL16 suppresses liver metastasis of colorectal cancer via augmentation of tumor-infiltrating natural killer T cells in a murine model. Oncol. Rep. 29, 975–982 (2013).
Zhao, L. et al. Recruitment of a myeloid cell subset (CD11b/Gr1(mid)) via CCL2/CCR2 promotes the development of colorectal cancer liver metastasis. Hepatology 57, 829–839 (2013).
Phillips, R. J. et al. The stromal derived factor-1/CXCL12-CXC chemokine receptor 4 biological axis in non-small cell lung cancer metastases. Am. J. Respir. Crit. Care Med. 167, 1676–1686 (2003).
Kajiyama, H. et al. Involvement of SDF-1α/CXCR4 axis in the enhanced peritoneal metastasis of epithelial ovarian carcinoma. Int. J. Cancer 122, 91–99 (2008).
US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT02179970.
Lok, E., Chung, A. S., Swanson, K. D. & Wong, E. T. Melanoma brain metastasis globally reconfigures chemokine and cytokine profiles in patient cerebrospinal fluid. Melanoma Res. 24, 120–130 (2014).
Erler, J. T. et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature 440, 1222–1226 (2006). This paper reports that LOX is a crucial component of metastatic colonization.
Canesin, G. et al. Lysyl oxidase-like 2 (LOXL2) and E47 EMT factor: novel partners in E-cadherin repression and early metastasis colonization. Oncogene 34, 951–964 (2015).
Hecht, J. R. et al. A phase II, randomized, double-blinded, placebo-controlled study of simtuzumab or placebo in combination with FOLFIRI for the second line treatment of metastatic KRAS mutant colorectal adenocarcinoma. J. Clin. Oncol. 33 (Suppl.) abstract 3537 (2015).
Erler, J. T. & Giaccia, A. J. Lysyl oxidase mediates hypoxic control of metastasis. Cancer Res. 66, 10238–10241 (2006).
Ostenfeld, M. S. et al. Cellular disposal of miR23b by RAB27-dependent exosome release is linked to acquisition of metastatic properties. Cancer Res. 74, 5758–5771 (2014).
Valencia, K. et al. miRNA cargo within exosome-like vesicle transfer influences metastatic bone colonization. Mol. Oncol. 8, 689–703 (2014).
Shimbo, K. et al. Exosome-formed synthetic microRNA-143 is transferred to osteosarcoma cells and inhibits their migration. Biochem. Biophys. Res. Commun. 445, 381–387 (2014).
Costa-Silva, B. et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 17, 816–826 (2015).
Peinado, H. et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 18, 883–891 (2012).
Le, M. T. N. et al. miR-200-containing extracellular vesicles promote breast cancer cell metastasis. J. Clin. Invest. 124, 5109–5128 (2014).
Zhang, L. et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature 527, 100–104 (2015). Exosomes contribute to metastatic colonization of the brain by altering astrocyte–tumour interactions.
Pan, Q. W. et al. Hepatic cell-to-cell transmission of small silencing RNA can extend the therapeutic reach of RNA interference (RNAi). Gut 61, 1330–1339 (2012).
Marleau, A. M., Chen, C. S., Joyce, J. A. & Tullis, R. H. Exosome removal as a therapeutic adjuvant in cancer. J. Transl Med. 10, 134 (2012).
US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT01779583 (2015).
US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT02393703 (2016).
US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT01550523 (2013).
US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT01159288 (2010).
US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT02439008 (2015).
Hu, G. H. et al. MTDH activation by 8q22 genomic gain promotes chemoresistance and metastasis of poor-prognosis breast cancer. Cancer Cell 15, 9–20 (2009).
Zhang, X. H. F. et al. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell 16, 67–78 (2009). SRC is a potential therapeutic target for dormant bone metastatic tumours.
Steeg, P. S., Camphausen, K. A. & Smith, Q. R. Brain metastases as preventive and therapeutic targets. Nat. Rev. Cancer 11, 352–363 (2011).
Fitzgerald, D. et al. Reactive glia are recruited by highly proliferative brain metastases of breast cancer and promote tumor cell colonization. Clin. Exp. Metast. 25, 799–810 (2008).
Lockman, P. R. et al. Heterogeneous blood–tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin. Cancer Res. 16, 5664–5678 (2010).
Kwon, E. D. et al. Elimination of residual metastatic prostate cancer after surgery and adjunctive cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) blockade immunotherapy. Proc. Natl Acad. Sci. USA 96, 15074–15079 (1999).
Iwai, Y. et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl Acad. Sci. USA 99, 12293–12297 (2002).
Strome, S. E. et al. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res. 63, 6501–6505 (2003).
Peggs, K. S., Quezada, S. A., Chambers, C. A., Korman, A. J. & Allison, J. P. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J. Exp. Med. 206, 1717–1725 (2009).
Parker, C. et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 369, 213–223 (2013).
Milenic, D. E., Baidoo, K. E., Kim, Y. S. & Brechbiel, M. W. Evaluation of cetuximab as a candidate for targeted alpha-particle radiation therapy of HER1-positive disseminated intraperitoneal disease. Mabs 7, 255–264 (2015). This paper reports a novel therapeutic strategy for established metastatic disease.
Hurvitz, S. A. et al. Phase II randomized study of trastuzumab emtansine versus trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J. Clin. Oncol. 31, 1157–1163 (2013).
Gould, S. E., Junttila, M. R. & De Sauvage, F. J. Translational value of mouse models in oncology drug development. Nat. Med. 21, 431–439 (2015).
DeRose, Y. S. et al. Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat. Med. 17, 1514–1520 (2011). This paper reports that PDXs provided new metastasis models that compared closely to patient outcomes.
Yi, B., Williams, P. J., Niewolna, M., Wang, Y. & Yoneda, T. Tumor-derived platelet-derived growth factor-BB plays a critical role in osteosclerotic bone metastasis in an animal model of human breast cancer. Cancer Res. 62, 917–923 (2002).
Gu, B., Espana, L., Mendez, O., Torregrosa, A. & Sierra, A. Organ-selective chemoresistance in metastasis from human breast cancer cells: inhibition of apoptosis, genetic variability and microenvironment at the metastatic focus. Carcinogenesis 25, 2293–2301 (2004).
Bhang, H. E. C. et al. Studying clonal dynamics in response to cancer therapy using high-complexity barcoding. Nat. Med. 21, 440–448 (2015).
Lange, J. & Ananworanich, J. The discovery and development of antiretroviral agents. Antiviral Ther. 19 (Suppl. 3), 5–14 (2014).
Weber, G. Why does cancer therapy lack effective anti-metastasis drugs? Cancer Lett. 328, 207–211 (2013).
Steeg, P. S. Perspective: the right trials. Nature 485, S58–S59 (2012).
Steeg, P. & Theodorescu, D. Metastasis: a therapeutic target for cancer. Nat. Clin. Pract. Oncol. 5, 206–219 (2008).
Li, S., Wang, N. & Brodt, P. Metastatic cells can escape the proapoptotic effects of TNF-α through increased autocrine IL-6/STAT3 signaling. Cancer Res. 72, 865–875 (2012).
Casar, B. et al. Blocking of CDCP1 cleavage in vivo prevents Akt-dependent survival and inhibits metastatic colonization through PARP1-mediated apoptosis of cancer cells. Oncogene 31, 3924–3938 (2012).
Woditschka, S. et al. DNA double-strand break repair genes and oxidative damage in brain metastasis of breast cancer. J. Natl Cancer Inst. 106, dju145 (2014).
Peng, Y. F. et al. Promoting colonization in metastatic HCC cells by modulation of autophagy. PLoS ONE 8, e74407 (2013).
Peng, Y. F. et al. Autophagy inhibition suppresses pulmonary metastasis of HCC in mice via impairing anoikis resistance and colonization of HCC cells. Autophagy 9, 2056–2068 (2013).
Sahni, S. et al. The metastasis suppressor, N-myc downstream-regulated gene 1 (NDRG1), inhibits stress-induced autophagy in cancer cells. J. Biol. Chem. 289, 9692–9709 (2014).
Kelber, J. A. et al. KRas induces a Src/PEAK1/ErbB2 kinase amplification loop that drives metastatic growth and therapy resistance in pancreatic cancer. Cancer Res. 72, 2554–2564 (2012).
Yang, Y. et al. Lifetime exposure to a soluble TGF-β antagonist protects mice against metastasis without adverse side effects. J. Clin. Invest. 109, 1607–1615 (2002). Preclinical validation of the prevention of metastasis and safety of potential TGFβ-directed therapeutics.
Kang, Y. et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 3, 537–549 (2003).
Sadok, A. et al. Rho kinase inhibitors block melanoma cell migration and inhibit metastasis. Cancer Res. 75, 2272–2284 (2015).
Tenbaum, S. P. et al. β-catenin confers resistance to PI3K and AKT inhibitors and subverts FOXO3a to promote metastasis in colon cancer. Nat. Med. 18, 892–991 (2012).
Yin, T. et al. Expression of snail in pancreatic cancer promotes metastasis and chemoresistance. J. Surg. Res. 141, 196–203 (2007).
Gupta, G. P. et al. ID genes mediate tumor reinitiation during breast cancer lung metastasis. Proc. Natl Acad. Sci. USA 104, 19506–19511 (2007). This paper identifies the early steps in metastatic colonization.
Chou, J. et al. GATA3 suppresses metastasis and modulates the tumour microenvironment by regulating microRNA-29b expression. Nat. Cell Biol. 15, 201–213 (2013).
Korpal, M. et al. Direct targeting of Sec23a by miR-200s influences cancer cell secretome and promotes metastatic colonization. Nat. Med. 17, 1101–1108 (2011).
Liu, Y. N. et al. Loss of androgen-regulated microRNA 1 activates SRC and promotes prostate cancer bone metastasis. Mol. Cell. Biol. 35, 1940–1951 (2015).
Tavazoie, S. F. et al. Endogenous human microRNAs that suppress breast cancer metastasis. Nature 451, 147–152 (2008).
Yang, F. et al. Repression of the long noncoding RNA-LET by histone deacetylase 3 contributes to hypoxia-mediated metastasis. Mol. Cell 50, 303–304 (2013).
Liao, J. Q. et al. Ovarian cancer spheroid cells with stem cell-like properties contribute to tumor generation, metastasis and chemotherapy resistance through hypoxia-resistant metabolism. PLoS ONE 9, e84941 (2014).
Bartucci, M. et al. TAZ is required for metastatic activity and chemoresistance of breast cancer stem cells. Oncogene 34, 681–690 (2015).
Malanchi, I. et al. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 481, 85–89 (2012). A POSTN-WNT mediated interaction between the microenvironment and tumour cells controls stemness and metastatic colonization.
Steeg, P. Metastasis suppressors alter the signal transduction of cancer cells. Nat. Rev. Cancer 3, 55–63 (2003).
Meehan, W. et al. The BRMS1 metastasis suppressor forms complexes with RBP1 and the mSin3 histone deacetylase complex and represses transcription. J. Biol. Chem. 279, 1562–1569 (2003).
Bandyopadhyay, S. et al. The Drg-1 gene suppresses tumor metastasis in prostate cancer. Cancer Res. 63, 1731–1736 (2003).
Horak, C. E. et al. Nm23-H1 suppresses metastasis by inhibiting expression of the lysophosphatidic acid receptor EDG2. Cancer Res. 67, 11751–11759 (2007).
Shtivelman, E. A link between metastasis and resistance to apoptosis of variant small cell lung carcinoma. Oncogene 14, 2167–2173 (1997).
Titus, B. et al. Endothelin axis is a target of the lung metastasis suppressor gene RhoGDI2. Cancer Res. 65, 7320–7327 (2005).
Szmulewitz, R. Z. et al. MKK4 suppresses metastatic colonization by multiple highly metastatic prostate cancer cell lines through a transient impairment in cell cycle progression. Int. J. Cancer 130, 509–520 (2012).
Fournier, P. et al. TGF-β signaling regulator PMEPA1 suppresses prostate cancer metastases to bone. Cancer Cell 27, 809–821 (2015).
Dai, J. L. et al. Bone morphogenetic protein-6 promotes osteoblastic prostate cancer bone metastases through a dual mechanism. Cancer Res. 65, 8274–8285 (2005).
Mohammad, K. S. et al. TGF-β-RI kinase inhibitor SD-208 reduces the development and progression of melanoma bone metastases. Cancer Res. 71, 175–184 (2011).
Ostapoff, K. T. et al. Neutralizing murine TGFβ R2 promotes a differentiated tumor cell phenotype and inhibits pancreatic cancer metastasis. Cancer Res. 74, 4996–5007 (2014).
Kang, Y. et al. Breast cancer bone metastasis mediated by the Smad tumor suppressor pathway. Proc. Natl Acad. Sci. USA 102, 13909–13914 (2005).
Yin, J. et al. TGF-β signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J. Clin. Invest. 103, 197–206 (1999).
Calon, A. et al. Dependency of colorectal cancer on a TGF-β-driven program in stromal cells for metastasis initiation. Cancer Cell 22, 571–584 (2012).
Stankic, M. et al. TGF-β-Id1 signaling opposes Twist1 and promotes metastatic colonization via a mesenchymal-to-epithelial transition. Cell Rep. 5, 1228–1242 (2013).
Pang, Y. L. et al. TGF-β signaling in myeloid cells is required for tumor metastasis. Cancer Discov. 3, 936–951 (2013).
Northey, J. J. et al. Distinct phosphotyrosine-dependent functions of the ShcA adaptor protein are required for transforming growth factor beta (TGFβ)-induced breast cancer cell migration, invasion, and metastasis. J. Biol. Chem. 288, 5210–5222 (2013).
Kohn, E. A. et al. Biological responses to TGF-β in the mammary epithelium show a complex dependency on Smad3 gene dosage with important implications for tumor progression. Mol. Cancer Res. 10, 1389–1399 (2012).
Xu, J. et al. 14-3-3 ζ turns TGF-β's function from tumor suppressor to metastasis promoter in breast cancer by contextual changes of Smad partners from p53 to Gli2. Cancer Cell 27, 177–192 (2015). 14-3-3-ζ interaction with p53 or GLI2 determines SMAD binding and TGFβ tumour suppressive versus pro-metastatic function.
Sato, M. et al. Differential proteome analysis identifies TGF-β-related pro-metastatic proteins in a 4T1 murine breast cancer model. PLoS ONE 10, e0126483 (2015).
Yang, L. TGF beta, a potent regulator of tumor microenvironment and host immune response: implication for therapy. Curr. Mol. Med. 10, 374–380 (2010).
Le, D. et al. PD-1 blockade in tumors with mismatch-repair deficiency. N.Engl. J. Med. 372, 2509–2520 (2015). Pembrolizumab, an immune checkpoint inhibitor, produced responses and extended PFS in mismatch repair-deficient metastatic colorectal cancer.
Larkin, J. et al. Combined novolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 373, 23–34 (2015). Phase III trial demonstrating superiority of two, versus one, immune checkpoint inhibitors as first-line treatment for stage III or IV melanoma.
Kaplan, R. N. et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438, 820–827 (2005). This paper reports that bone marrow-derived cells arrive in potential metastatic sites before tumour cells and begin to modify the microenvironment.
Yan, H. H. et al. Gr-1+CD11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Res. 70, 6139–6149 (2010).
Hiratsuka, S., Watanabe, A., Aburatani, H. & Maru, Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat. Cell Biol. 8, 1369–1375 (2006).
Hiratsuka, S. et al. The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase. Nat. Cell Biol. 10, 1349–1355 (2008).
Cox, T. R. et al. The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase. Nature 522, 106–110 (2015).
Chafe, S. C. et al. Carbonic anhydrase IX promotes myeloid-derived suppressor cell mobilization and establishment of a metastatic niche by stimulating G-CSF production. Cancer Res. 75, 996–1008 (2015).
Sceneay, J. et al. Primary tumor hypoxia recruits CD11b+/Ly6C(med)/Ly6G+ immune suppressor cells and compromises NK cell cytotoxicity in the premetastatic niche. Cancer Res. 72, 3906–3911 (2012).
Fong, M. Y. et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat. Cell Biol. 17, 183–194 (2015).
Gonzalez-Zubeldia, I. et al. Co-migration of colon cancer cells and CAFs induced by TGF beta(1) enhances liver metastasis. Cell Tissue Res. 359, 829–839 (2015).
Sawada, S., Murakami, K., Murata, J., Tsukada, K. & Saiki, I. Accumulation of extracellular matrix in the liver induces high metastatic potential of hepatocellular carcinoma to the lung. Int. J. Oncol. 19, 65–70 (2001).
Kitamura, T. et al. Inactivation of chemokine (C-C motif) receptor 1 (CCR1) suppresses colon cancer liver metastasis by blocking accumulation of immature myeloid cells in a mouse model. Proc. Natl Acad. Sci. USA 107, 13063–13068 (2010).
Kim, S. et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature 457, 102–108 (2009).
Liu, L. et al. Reductions in myeloid-derived suppressor cells and lung metastases using AZD4547 treatment of a metastatic murine breast tumor model. Cell. Physiol. Biochem. 33, 633–645 (2014).
Li, H. et al. Activation of PPARγ in myeloid cells promotes lung cancer progression and metastasis. PLoS ONE 6, e28133 (2011).
Sawant, A. & Ponnazhagan, S. Myeloid-derived suppressor cells as osteoclast progenitors: a novel target for controlling osteolytic bone metastasis. Cancer Res. 73, 4606–4610 (2013).
Chen, Q., Zhang, X. H. F. & Massague, J. Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs. Cancer Cell 20, 538–549 (2011). This paper reports that tumour VCAM1 interaction with macrophages provides a pro-survival signal in metastatic colonization.
Chapon, M. et al. Progressive upregulation of PD-1 in primary and metastatic melanomas associated with blunted TCR signaling in infiltrating T lymphocytes. J. Invest. Dermatol. 131, 1300–1307 (2011). This study shows that immune checkpoint expression varies in metastases.
Spranger, S. et al. Up-regulation of PD-L1, IDO, and T-regs in the melanoma tumor microenvironment is driven by CD8+ T cells. Sci. Transl Med. 5, 200ra116 (2013).
Abiko, K. et al. IFN-gamma from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br. J. Cancer 112, 1501–1509 (2015). This paper reports that IFNγ regulates PDL1 immune checkpoint expression and function in peritoneal colonization of ovarian cancer.
Coffelt, S. B. et al. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature 522, 345–348 (2015).
Olkhanud, P. B. et al. Breast cancer lung metastasis requires expression of chemokine receptor CCR4 and regulatory T cells. Cancer Res. 69, 5996–6004 (2009).
Chen, L. M. et al. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat. Commun. 5, 5241 (2014).
Donnem, T. et al. Vessel co-option in primary human tumors and metastases: an obstacle to effective anti-angiogenic treatment? Cancer Med. 2, 427–436 (2013).
Bos, P. et al. Genes that mediate breast cancer metastasis to the brain. Nature 459, 1005–1010 (2009).
Carbonell, W. S., Ansorge, O., Sibson, N. & Muschel, R. The vascular basement membrane as “soil'' in brain metastasis. PLoS ONE 4, 5241 (2009). Identification of the perivascular niche as a crucial microenvironment for metastatic colonization of the brain.
Xing, F. et al. Reactive astrocytes promote the metastatic growth of breast cancer stem-like cells by activating Notch signalling in brain. EMBO Mol. Med. 5, 384–396 (2013).
Gril, B. et al. Pazopanib inhibits the activation of PDGFR β-expressing astrocytes in the brain metastatic microenvironment of breast cancer cells. Am. J. Pathol. 182, 2368–2379 (2013).
Noda, M. et al. IL-6 receptor is a possible target against growth of metastasized lung tumor cells in the brain. Int. J. Mol. Sci. 14, 515–526 (2013).
Sartorius, C. et al. Estrogen promotes the brain metastatic colonization of triple negative breast cells via an astrocyte-mediated paracrine mechanism. Oncogene, http://dx.doi.org/10.1038/onc.2015.353 (2016).
Binder, C. et al. Relaxins enhance growth of spontaneous murine breast cancers as well as metastatic colonization of the brain. Clin. Exp. Metastasis 31, 57–65 (2014).
Silver, D. J. et al. Chondroitin sulfate proteoglycans potently inhibit invasion and serve as a central organizer of the brain tumor microenvironment. J. Neurosci. 33, 15603–15617 (2013).
Louie, E. et al. Neurotrophin-3 modulates breast cancer cells and the microenvironment to promote the growth of breast cancer brain metastasis. Oncogene 32, 4064–4077 (2013).
Pukrop, T. et al. Microglia promote colonization of brain tissue by breast cancer cells in a Wnt-dependent way. Glia 58, 1477–1489 (2010).
Liu, Y. et al. Premetastatic soil and prevention of breast cancer brain metastasis. Neuro-Oncol. 15, 891–903 (2013).
Liu, Y. et al. Expression of antigen processing and presenting molecules in brain metastasis of breast cancer. Cancer Immunol. Immunother. 61, 789–801 (2012).
Chen, E. I. et al. Adaptation of energy metabolism in breast cancer brain metastases. Cancer Res. 67, 1472–1486 (2007).
Valiente, M. et al. Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell 156, 1002–1016 (2014).
Fitzgerald, D. P. et al. Opposing effects of pigment epithelium-derived factor on breast cancer cell versus neuronal survival: implication for brain metastasis and metastasis-induced brain damage. Cancer Res. 72, 144–153 (2012).
Bock, F. et al. Bevacizumab as a potent inhibitor of inflammatory corneal angiogenesis and lymphangiogenesis. Invest. Ophthalmol. Vis. Sci. 48, 2545–2552 (2007).
Ferrara, N., Hillan, K. J., Gerber, H. P. & Novotny, W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat. Rev. Drug Discov. 3, 391–400 (2004).
Bauerle, T. et al. Bevacizumab inhibits breast cancer-induced osteolysis, surrounding soft tissue metastasis, and angiogenesis in rats as visualized by VCT and MRI. Neoplasia 10, 511–520 (2008).
Ninomiya, S. et al. Effect of bevacizumab, a humanized monoclonal antibody to vascular endothelial growth factor, on peritoneal metastasis of MNK-45P human gastric cancer in mice. J. Surg. Res. 154, 196–202 (2009).
Burke, P. A. et al. Cilengitide targeting of αvβ3 integrin receptor synergizes with radioimmunotherapy to increase efficacy and apoptosis in breast cancer xenografts. Cancer Res. 62, 4263–4272 (2002).
Kurozumi, K., Ichikawa, T., Onishi, M., Fujii, K. & Date, I. Cilengitide treatment for malignant glioma: current status and future direction. Neurol. Med.-Chirurg. 52, 539–547 (2012).
Shah, N. P. et al. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science 305, 399–401 (2004).
Dunn, E. F. et al. Dasatinib sensitizes KRAS mutant colorectal tumors to cetuximab. Oncogene 30, 561–574 (2011).
Arcaroli, J. J. et al. Gene array and fluorescence in situ hybridization biomarkers of activity of saracatinib (AZD0530), a Src inhibitor, in a preclinical model of colorectal cancer. Clin. Cancer Res. 16, 4165–4177 (2010).
Levitt, J. M., Yamashita, H., Jian, W., Lerner, S. P. & Sonpavde, G. Dasatinib is preclinically active against Src-overexpressing human transitional cell carcinoma of the urothelium with activated Src signaling. Mol. Cancer Ther. 9, 1128–1135 (2010).
Morton, J. P. et al. Dasatinib inhibits the development of metastases in a mouse model of pancreatic ductal adenocarcinoma. Gastroenterology 139, 292–303 (2010).
Chan, C. M. et al. Targeted inhibition of Src kinase with dasatinib blocks thyroid cancer growth and metastasis. Clin. Cancer Res. 18, 3580–3591 (2012).
Yamaguchi, H. et al. Saracatinib impairs the peritoneal dissemination of diffuse-type gastric carcinoma cells resistant to Met and fibroblast growth factor receptor inhibitors. Cancer Sci. 105, 528–536 (2014).
Zhang, S. Y. et al. Src family kinases as novel therapeutic targets to treat breast cancer brain metastases. Cancer Res. 73, 5764–5774 (2013).
Trevino, J. G. et al. Inhibition of Src expression and activity inhibits tumor progression and metastasis of human pancreatic adenocarcinoma cells in an orthotopic nude mouse model. Am. J. Pathol. 168, 962–972 (2006).
Hingorani, P., Zhang, W. D., Gorlick, R. & Kolb, E. A. Inhibition of Src phosphorylation alters metastatic potential of osteosarcoma in vitro but not in vivo. Clin. Cancer Res. 15, 3416–3422 (2009).
Saltz, L. B. et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J. Clin. Oncol. 26, 2013–2019 (2008).
Hurwitz, H. et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 350, 2335–2342 (2004).
Yang, J. C. et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N. Engl. J. Med. 349, 427–434 (2003).
Gilbert, M. R. et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N. Engl. J. Med. 370, 699–708 (2014).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author has received research grants from Sanofi and Genentech.
Related links
Glossary
- Standard of care
-
(SOC). Also known as best practice, treatment for each type and stage of cancer that is accepted in general practice by health care professionals, and iterated in guidelines such as those of the US National Comprehensive Cancer Network.
- Metastatic colonization
-
The progressive growth of a lesion in a foreign location.
- Invasion
-
Cancer cells traverse normal tissues in groups or as single cells, using reversible adhesion, proteolytic destruction and motility.
- Genomic instability
-
A state of high frequency of mutations in a cell, including nucleic acid sequences, chromosomal rearrangements and aneuploidy.
- Localized disease
-
In the clinic, disease that is limited to the tissue or organ in which it began.
- Regional disease
-
Cancer that has grown beyond the original tumour and spread to nearby lymph nodes or tissues.
- Overall survival
-
(OS). The length of time, either from disease diagnosis or the beginning of treatment, until death.
- Progression-free survival
-
(PFS). A metric of patient response to therapy, measured from the time of treatment initiation or clinical trial enrolment until either detectable lesions increase, based on standard measurement criteria, or the patient dies.
- Adjuvant trials
-
Clinical trials to test whether an additional treatment after primary therapy will lower the risk of cancer recurrence.
- Pathological complete response
-
(pCR). The absence of residual invasive tumour cells by microscopic examination of resected tissue after neoadjuvant therapy.
- Neoadjuvant trial
-
In cancer, a trial testing a potential therapy before the 'definitive' treatment, such as primary tumour surgery.
- Half-life
-
In pharmacology, the time it takes for a compound to fall to one-half of its initial steady-state level.
- G-protein-coupled receptors
-
A family of integral membrane receptors that sense extracellular signals and activate intracellular signalling by binding to G proteins.
- Focal adhesion kinase
-
(FAK). Cytosolic non-receptor protein kinase typically linking extracellular matrix with the actin network, regulating cell adhesion, viability and spreading.
- Stable disease
-
A metric of patient response to therapy, in which measurable lesions are neither increasing nor decreasing based on standard measurement criteria.
- Maximum tolerated dose
-
(MTD). The highest dose of a drug or treatment that does not cause unacceptable side effects.
- Extravasation
-
In metastasis, the movement of tumour cells out of the circulatory system into surrounding tissues.
- Stem or tumour-initiating cell
-
A cell found within a cancer that is tumorigenic and can differentiate into one of several cell types found within the tumour.
- Anoikis
-
A form of programmed cell death induced by anchorage-dependent cells detaching from an extracellular matrix.
- Neoantigens
-
Peptides absent from the normal genome, caused by somatic mutations.
- Myofibroblasts
-
Cells with attributes of fibroblasts and smooth muscle cells that are activated to participate in wound repair.
- Mismatch repair
-
A form of DNA repair that corrects erroneous misincorporation of bases during replication, and other insertions, deletions and DNA damage.
- Double strand break repair
-
Repair of hazardous lesions in which both strands of DNA are broken, by non-homologous end joining or homologous recombination repair.
- Nonspecific immunity
-
Also called innate immunity, host responses to pathogens or tumour cells that do not provide long-term memory or protection.
- Adaptive immunity
-
Part of the immune system by which memory is acquired after an initial response to a specific antigen.
- Natural killer (NK) cells
-
Lymphocytes that are cytotoxic for virally infected or tumour cells, without the need for major histocompatibility complex (MHC) and T cell receptor signalling.
- Minimal residual disease
-
In leukaemia, a low level of tumour cells or their products in patients apparently treated successfully, detectable only with molecular markers.
- Astrocytes
-
Star-shaped cells in the brain and spinal cord that maintain the blood–brain barrier, provide nutrients, maintain ion balance and assist in injury repair.
- Microglia
-
Resident macrophage-like cells of the brain and spinal cord.
- Neuroinflammatory response
-
Inflammation of the central nervous system characterized by activation of endothelial and glial cells, cytokines and oedema.
- α-particle
-
A particle for radiation therapy consisting of a helium nucleus with high energy and low penetrance.
- Xenografts
-
In cancer, cells or tissues transplanted from one species to another, often human cancer cells into immunodeficient mice.
- Genetically engineered mouse (GEM) models
-
Mouse models in which the genome has been altered, including transgenes and targeted mutations (knockouts or knockins).
- Patient-derived xenografts
-
(PDXs). Patient tumour tissues implanted directly into immunodeficient mice.
- Micrometastases
-
Metastatic lesions that are too small for conventional detection.
Rights and permissions
About this article
Cite this article
Steeg, P. Targeting metastasis. Nat Rev Cancer 16, 201–218 (2016). https://doi.org/10.1038/nrc.2016.25
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc.2016.25
This article is cited by
-
High-fat diet promotes prostate cancer metastasis via RPS27
Cancer & Metabolism (2024)
-
Kv3.4 regulates cell migration and invasion through TGF-β-induced epithelial–mesenchymal transition in A549 cells
Scientific Reports (2024)
-
Unveiling breast cancer metastasis through an advanced X-ray imaging approach
Scientific Reports (2024)
-
KLF5 regulates actin remodeling to enhance the metastasis of nasopharyngeal carcinoma
Oncogene (2024)
-
Linking cell mechanical memory and cancer metastasis
Nature Reviews Cancer (2024)