Abstract
Carbon monoxide (CO) is a key gaseous signaling molecule in living cells and organisms. This protocol illustrates the synthesis of a highly sensitive Nile Red (NR)–Pd-based fluorescent probe, NR-PdA, and its applications for detecting endogenous CO in tissue culture cells, ex vivo organs, and zebrafish embryos. In the NR-PdA synthesis process, 3-diethylamine phenol reacts with sodium nitrite in the acidic condition to afford 5-(diethylamino)-2-nitrosophenol hydrochloride (compound 1), which is further treated with 1-naphthalenol at a high temperature to provide the NR dye via a cyclization reaction. Finally, NR is reacted with palladium acetate to obtain the desired Pd-based fluorescent probe NR-PdA. NR-PdA possesses excellent two-photon excitation and near-IR emission properties, high stability, low background fluorescence, and a low detection limit. In addition to the chemical synthesis procedures, we provide step-by-step procedures for imaging endogenous CO in RAW 264.7 cells, mouse organs ex vivo, and live zebrafish embryos. The synthesis process for the probe requires ∼4 d, and the biological imaging experiments take ∼14 d.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Bauer, I. & Pannen, B.H.J. Bench-to-bedside review: carbon monoxide--from mitochondrial poisoning to therapeutic use. Crit. Care 13, 220 (2009).
Levitt, D.G. & Levitt, M.D. Carbon monoxide: a critical quantitative analysis and review of the extent and limitations of its second messenger function. Clin. Pharmacol. 7, 37–56 (2015).
Wu, L. & Wang, R. Carbon monoxide: endogenous production, physiological functions, and pharmacological applications. Pharmacol. Rev. 57, 585–630 (2005).
Ryter, S.W. & Choi, A.M.K. Carbon monoxide: present and future indications for a medical gas. J. Intern. Med. 28, 123–140 (2013).
Ibrahim, M.Y., El-Sayed, S.A., Abdel-Hakim, S.M., Hassan, M.K.A. & Aziz, N.M. The effect of induction of endogenous CO by heme-oxygenase inducer, hemin versus heme-oxygenase blocker, zinc mesoporphyrin on gastric secretion and ulceration under different conditions in adult male albino rats. Bratisl. Lek. Listy 115, 319–329 (2014).
Dunn, L.L. et al. New insights into intracellular locations and functions of heme oxygenase-1. Antioxid. Redox Signal. 20, 1723–1742 (2014).
Nassour, I. et al. Carbon monoxide protects against hemorrhagic shock and resuscitation–induced microcirculatory injury and tissue injury. Shock 43, 166–171 (2015).
Patel, R. et al. Inhalation of carbon monoxide reduces skeletal muscle injury after hind limb ischemia-reperfusion injury in mice. Am. J. Surg. 203, 488–495 (2012).
Motterlini, R. & Otterbein, L.E. The therapeutic potential of carbon monoxide. Nat. Rev. Drug Discov. 9, 728–43 (2010).
Knauert, M., Vangala, S., Haslip, M. & Lee, P.J. Therapeutic applications of carbon monoxide. Oxid. Med. Cell. Longev. 11, 1–11 (2013).
Ryter, S.W. & Choi, A.M.K. Heme oxygenase-1/carbon monoxide: from metabolism to molecular therapy. Am. J. Resp. Cell Mol. 41, 251–260 (2009).
Nakao, A., Kaczorowski, D.J., Sugimoto, R., Billiar, T.R. & McCurry, K.R. Application of heme oxygenase-1, carbon monoxide and biliverdin for the prevention of intestinal ischemia/reperfusion injury. J. Clin. Biochem. Nutr. 42, 78–88 (2008).
D'Amico, G., Lam, F., Hagen, T. & Moncada, S. Inhibition of cellular respiration by endogenously produced carbon monoxide. J. Cell Sci. 119, 2291–2298 (2006).
Wang, D. et al. A click-and-release approach to CO prodrugs. Chem. Commun. 50, 15890–15893 (2014).
Motterlini, R. Carbon monoxide-releasing molecules (CO-RMs): vasodilatory, anti-ischaemic and anti-inflammatory activities. Biochem. Soc. 35, 1142–1146 (2007).
Motterlini, R. et al. Carbon monoxide-releasing molecules. Circ. Res. 90, e17–e24 (2002).
He, L., Dong, B., Liu, Y. & Lin, W. Fluorescent chemosensors manipulated by dual/triple interplaying sensing mechanisms. Chem. Soc. Rev. 45, 6449–6461 (2016).
Chen, H., Dong, B., Tang, Y. & Lin, W. A unique 'integration' strategy for the rational design of optically tunable near-infrared fluorophores. Acc. Chem. Res. 50, 1410–1422 (2017).
Chan, J., Dodani, S.C. & Chang, C.J. Reaction-based small-molecule fluorescent probes for chemoselective bioimaging. Nat. Chem. 4, 973–984 (2012).
Yin, J., Hu, Y. & Yoon, J. Fluorescent probes and bioimaging: alkali metals, alkaline earth metals and pH. Chem. Soc. Rev. 44, 4619–4644 (2015).
Kim, J.S. & Quang, D.T. Calixarene-derived fluorescent probes. Chem. Rev. 38, 3780–3799 (2007).
Basudhar, D. et al. Biological signaling by small inorganic molecules. Coord. Chem. Rev. 306, 708–723 (2016).
Yusop, R.M., Unciti-Broceta, A., Johansson, E.M.V., Sánchez-Martín, R.M. & Bradley, M. Palladium-mediated intracellular chemistry. Nat. Chem. 3, 239–243 (2011).
Weiss, J.T. et al. Development and bioorthogonal activation of palladium-labile prodrugs of gemcitabine. J. Med. Chem. 57, 5395–5404 (2014).
Michel, B.W., Lippert, A.R. & Chang, C.J. A reaction-based fluorescent probe for selective imaging of carbon monoxide in living cells using a palladium-mediated carbonylation. J. Am. Chem. Soc. 134, 15668–15671 (2012).
Streu, C. & Meggers, E. Ruthenium-induced allylcarbamate cleavage in living cells. Angew. Chem. Int. Ed. 45, 5645–5648 (2006).
Heinemann, S.H., Hoshi, T., Westerhausen, M. & Schiller, A. Carbon monoxide - physiology, detection and controlled release. Chem. Commun. 50, 3644–3660 (2014).
Li, Y. et al. Fluorescent probe based on azobenzene-cyclopalladium for the selective imaging of endogenous carbon monoxide under hypoxia conditions. Anal. Chem. 88, 11154–11159 (2016).
Zheng, K., Lin, W., Tan, L., Chen, H. & Cui, H. A unique carbazole-coumarin fused two-photon platform: development of a robust two-photon fluorescent probe for imaging carbon monoxide in living tissues. Chem. Sci. 5, 3439–3448 (2014).
Feng, W., Liu, D., Feng, S. & Feng, G. Readily available fluorescent probe for carbon monoxide imaging in living cells. Anal. Chem. 88, 10648–10653 (2016).
Feng, S., Liu, D., Feng, W. & Feng, G. Allyl fluorescein ethers as promising fluorescent probes for carbon monoxide imaging in living cells. Anal. Chem. 89, 3754–3760 (2017).
Feng, W., Hong, J. & Feng, G. Colorimetric and ratiometric fluorescent detection of carbon monoxide in air, aqueous solution, and living cells by a naphthalimide-based probe. Sens. Actuators B-Chem. 251, 389–395 (2017).
Feng, W., Liu, D., Zhai, Q. & Feng, G. Lighting up carbon monoxide in living cells by a readily available and highly sensitive colorimetric and fluorescent probe. Sens. Actuators B-Chem. 240, 625–630 (2017).
Feng, W. & Feng, G. A readily available colorimetric and near-infrared fluorescent turn-on probe for detection of carbon monoxide in living cells and animals. Sens. Actuators B-Chem. 255, 2314–2320 (2018).
Liu, K., Kong, X., Ma, Y. & Lin, W. Rational design of a robust fluorescent probe for the detection of endogenous carbon monoxide in living zebrafish embryos and mouse tissue. Angew. Chem. Int. Ed. 56, 13489–13492 (2017).
Owens, E.O. Endogenous carbon monoxide production in disease. Clin. Biochem. 43, 1183–1188 (2010).
Park, S.S. et al. The real-time in vivo electrochemical measurement of nitric oxide and carbon monoxide release upon direct epidural electrical stimulation of the rat neocortex. Analyst 140, 3415–3421 (2015).
Ha, Y., Sim, J., Lee, Y. & Suh, M. Insertable fast-response amperometric NO/CO dual microsensor: study of neurovascular coupling during acutely induced seizures of rat brain cortex. Anal. Chem. 88, 2563–2569 (2016).
Cao, Y. et al. Highly selective detection of carbon monoxide in living cells by palladacycle carbonylation-based surface enhanced Raman spectroscopy nanosensors. Anal. Chem. 87, 9696–9701 (2015).
Park, S.S., Kim, J. & Lee, Y. Improved electrochemical microsensor for the real-time simultaneous analysis of endogenous nitric oxide and carbon monoxide generation. Anal. Chem. 84, 1792–1796 (2012).
Coburn, R.F. The measurement of endogenous carbon monoxide production. J. Appl. Physiol. 112, 1949–1955 (2012).
Kitagishi, H. et al. Feedback response to selective depletion of endogenous carbon monoxide in the blood. J. Am. Chem. Soc. 138, 5417–5425 (2016).
Antaris, A.L. et al. A small-molecule dye for NIR-II imaging. Nat. Mater. 15, 235–242 (2015).
Hoebe, R.A. et al. Controlled light-exposure microscopy reduces photobleaching and phototoxicity in fluorescence live-cell imaging. Nat. Biotechnol. 25, 249–253 (2007).
Ryter, S.W. & Choi, A.M.K. Targeting heme oxygenase-1 and carbon monoxide for therapeutic modulation of inflammation. Transl. Res. 167, 7–34 (2016).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (NSFC; 21472067, 21672083, and 61605060), Natural Science Foundation (NSF) of Shandong Province (ZR2015PB016, ZR2017PH051), the Taishan Scholar Foundation (TS 201511041), and the startup fund of the University of Jinan (309-10004, 160100137).
Author information
Authors and Affiliations
Contributions
W.L. directed the research and conceived the project with K.L. and X.K. K.L. and X.K. contributed equally to this work. The experiments were performed by K.L., X.K, and Y.M. All the authors analyzed the data and contributed to the manuscript writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
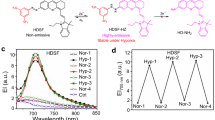
Supplementary Figure 1 Spectroscopic titration changes of CO fluorescent probe NR-PdA.
The fluorescence titration of 2 μM NR-PdA towards CO (0-200 μM) after 30 min in PBS (10 mM, pH 7.4, 5% DMSO). λex = 580 nm, λem = 600-800 nm. Inset: the fluorescence intensity changes at 660 nm of NR-PdA towards different ratios of CO versus probe. λex = 580 nm, λem = 660 nm. Reproduced with permission from ref. 35, Wiley.
Supplementary Figure 2 The linear curve of fluorescence changes of NR-PdA toward various concentrations of CO.
The detection limit determination was studied with 0.5 μM NR-PdA in PBS (10 mM, pH 7.4, 5% DMSO). The fluorescence changes at 660 nm of NR-PdA interacted with various concentrations of CO for 30 min. λex = 580 nm, λem = 660 nm. Reproduced with permission from ref. 35, Wiley.
Supplementary Figure 3 The photostability study of NR-PdA.
The fluorescence intensity changes at 660 nm of 2 μM NR-PdA in PBS (10 mM, pH 7.4, 5% DMSO) for 600 min excited at 360 nm and 580 nm respectively. Reproduced with permission from ref. 35, Wiley.
Supplementary Figure 4 The spectroscopic selectivity of the CO fluorescent probe NR-PdA.
The spectroscopic responses of 2 μM NR-PdA towards various analytes for 30 min in PBS (10 mM, pH 7.4, 5% DMSO). Analytes given are 200 μM H2O2, OH·, CH3COOOH, ClO−, TBHP, t−BuOOH, NO, NO2−, ONOO−, Cys, HCy, GSH, HS−, SO32−, S2O32−, 1O2, O2−, Vc, Fe2+, CO. (a) Fluorescence curves of NR-PdA in the presence of various analytes, λex = 580 nm, λem = 600-800 nm. (b) Fluorescence intensities of NR-PdA at 660 nm in the presence of various analytes excited at 580 nm. Reproduced with permission from reference 1 in Supporting Information. Adapted with permission from ref. 35, Wiley.
Supplementary Figure 5 Cell cytotoxicity study of NR-PdA in HeLa and RAW 264.7 cells.
Cell viability of HeLa and RAW 264.7 cells treated with different concentrations (0-20 μM) of NR-PdA for 24 h. Error bars denote standard deviation (±S.D.). n = 3, the statistical analysis was obtained from three separate measurements. Adapted with permission from ref. 35, Wiley.
Supplementary Figure 6 One- and two-photon images of exogenous CO in HeLa cells treated with NR-PdA and CO.
One- and two-photon fluorescence images of exogenous CO in HeLa cells by NR-PdA with time. (a) Fluorescence images of cells treated with 5 μM NR-PdA for 30 min (left panel), then cells loaded with 20 μM CORM-2 for imaging after co-incubation for 10 min, 20 min, 30 min, 90 min, respectively (right panel). (b) The corresponding quantification of fluorescence intensities from the cells loaded with NR-PdA at different time relative to the cell fluorescence absence of CORM-2 obtained by one-photon excitation. (c) The corresponding quantification of fluorescence intensities from the cells loaded with NR-PdA in the presence of CORM-2 at different time relative to the cell fluorescence absence of CORM-2 obtained by two-photon excitation. The statistical analysis is carried out from three separate measurements. The data in (b, c) show the individual data points obtained from three separate measurements for the calculation of the mean and SD values. Error bars denote standard deviation (±S.D.), n = 3. One-photon imaging: λex = 561 nm; λem = 570-620 nm. Two-photon imaging: λex = 760 nm; λem = 570-620 nm. Scale bar: 20 μm
Supplementary Figure 7 Image of compound 1 on a thin-layer chromatography plate.
Compound 1 was spotted on a thin-layer chromatography plate by point capillary, then eluted by a mixture of dichloromethane and methanol (60/1, vol/vol).
Supplementary Figure 8 Images of NR on a thin-layer chromatography plate.
NR was spotted on a thin-layer chromatography plate by point capillary, then eluted by a mixture of dichloromethane and methanol (50/1, vol/vol). (a) Image under white light, (b) Image under 365 nm light of a hand-hold UV lamb.
Supplementary Figure 9 Images of NR-PdA on a thin-layer chromatography plate.
(a) NR-PdA was spotted on a thin-layer chromatography plate by point capillary, then eluted by a mixture of dichloromethane and methanol (20/1, vol/vol). (b) NR-PdA was spotted on a thin-layer chromatography plate by point capillary, then eluted by a mixture of n−butanol/acetic acid/H2O (4/1/1, vol/vol/vol).
Supplementary Figure 10 One- and two-photon images of endogenous CO in RAW264.7 cells treated with NR-PdA.
One- and two-photon fluorescence images of endogenous CO in RAW 264.7 cells by NR-PdA with time. (a) Fluorescence images of cells pre-treated in a hypoxia condition (37 °C, 5% CO2, 2% O2) for 24 h, then treated with 5 μM NR-PdA for incubation 10, 20, 30, and 60 min respectively. (b) The corresponding quantification of fluorescence intensities from the cells loaded with NR-PdA at different time relative to the cell fluorescence absence of NR-PdA obtained by one-photon excitation. (c) The corresponding quantification of relative fluorescence intensities from the cells loaded with NR-PdA at different time relative to the cell fluorescence absence of NR-PdA obtained by two-photon excitation. The statistical analysis is carried out from three separate measurements. The data in (b, c) show the individual data points obtained from three separate measurements for the calculation of the mean and SD values. Error bars denote standard deviation (±S.D.), n = 3. One-photon imaging: λex = 561 nm; λem = 570-620 nm. Two-photon imaging: λex = 760 nm; λem = 570-620 nm. Scale bar: 20 μm
Supplementary Figure 11 Quantification fluorescence intensities of endogenous CO using NR-PdA in various organs.
The quantification fluorescence intensities of endogenous CO using 10 μM NR-PdA in various mouse organs at different time (20, 40 and 120 min) using an in vivo imaging system. (a) The quantification fluorescence intensities of different organs from the mice received 100 μL LPS (0.5 mg/mL) by an i.p. injection for 4 days. (b) The quantification fluorescence intensities of different organs from the mice received 100 μL Hemin (1 mM) by an i.p. injection for 4 days. (c) The quantification fluorescence intensities of different organs from the mice received 100 μL ZnPP (1 mM) by an i.p. injection for 4 days. The statistical analysis is carried out from three separate measurements. The data in (a, b, c) show the individual data points obtained from three separate measurements for the calculation of the mean and SD values. Error bars denote standard deviation (±S.D.), n = 3. Adapted with permission from ref. 35, Wiley.
Reference
Liu, K., Kong, X., Ma, Y. & Lin, W. (2017) Rational design of a robust fluorescent probe for the detection of endogenous carbon monoxide in living zebrafish embryos and mouse tissue. Angew. Chem. Int. Ed. 56, 13489-13492.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–11, and Supplementary Tables 1 and 2. (PDF 1515 kb)
Rights and permissions
About this article
Cite this article
Liu, K., Kong, X., Ma, Y. et al. Preparation of a Nile Red–Pd-based fluorescent CO probe and its imaging applications in vitro and in vivo. Nat Protoc 13, 1020–1033 (2018). https://doi.org/10.1038/nprot.2018.013
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2018.013
This article is cited by
-
Ultrasound-driven self-decomposition porphyrins as metal-free CO precursors for gas and sonodynamic synergistic therapy
Science China Chemistry (2024)
-
BODIPY Based OFF-ON Fluorescent Probe for Endogenous Carbon Monoxide Imaging in Living Cells
Journal of Fluorescence (2023)
-
Luminescent Metal Complexes as Emerging Tools for Lipid Imaging
Topics in Current Chemistry (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.