Abstract
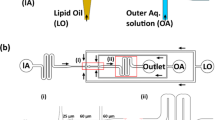
In this protocol, we describe a recently developed on-chip microfluidic method to form monodisperse, cell-sized, unilamellar, and biocompatible liposomes with excellent encapsulation efficiency. Termed octanol-assisted liposome assembly (OLA), it resembles bubble-blowing on a microscopic scale. Hydrodynamic flow focusing of two immiscible fluid streams (an aqueous phase and a lipid-containing 1-octanol phase) by orthogonal outer aqueous streams gives rise to double-emulsion droplets. As the lipid bilayer assembles along the interface, each emulsion droplet quickly evolves into a liposome and a 1-octanol droplet. OLA has several advantages as compared with other on-chip techniques, such as a very fast liposome maturation time (a few minutes), a relatively straightforward and completely on-chip setup, and a biologically relevant liposome size range (5–20 μm). Owing to the entire approach being on-chip, OLA enables high-throughput liposome production (typical rate of tens of Hz) using low sample volumes (∼10 μl). For prolonged on-chip experimentation, liposomes are subsequently purified by removing the 1-octanol droplets. For device fabrication, a reusable silicon template is produced in a clean room facility using electron-beam lithography followed by dry reactive ion etching, which takes ∼3 h. The patterned silicon template is used to prepare polydimethylsiloxane (PDMS)-based microfluidic devices in the wet lab, followed by a crucial surface treatment; the whole process takes ∼2 d. Liposomes can be produced in ∼1 h and further manipulated, depending on the experimental setup. OLA offers an ideal microfluidic platform for diverse bottom-up biotechnology studies by enabling creation of synthetic cells, microreactors and bioactive cargo delivery systems, and also has potential as an analytical tool.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Balaram, P. Synthesizing life. Curr. Sci. 85, 1509–1510 (2003).
Pohorille, A. & Deamer, D. Artificial cells: prospects for biotechnology. Trends Biotechnol. 20, 123–128 (2002).
Deamer, D. On the origin of systems. Systems biology, synthetic biology and the origin of life. EMBO Rep. 10, S1–S4 (2009).
Walde, P. Building artificial cells and protocell models: experimental approaches with lipid vesicles. BioEssays 32, 296–303 (2010).
Dzieciol, A.J. & Mann, S. Designs for life: protocell models in the laboratory. Chem. Soc. Rev. 41, 79 (2012).
Shang, L., Cheng, Y. & Zhao, Y. Emerging droplet microfluidics. Chem. Rev. 117, 7964–8040 (2017).
van Swaay, D. & deMello, A. Microfluidic methods for forming liposomes. Lab Chip 13, 752–67 (2013).
Stano, P., Carrara, P., Kuruma, Y., Pereira de Souza, T. & Luisi, P.L. Compartmentalized reactions as a case of soft-matter biotechnology: synthesis of proteins and nucleic acids inside lipid vesicles. J. Mater. Chem. 21, 18887 (2011).
Deshpande, S., Caspi, Y., Meijering, A.E. & Dekker, C. Octanol-assisted liposome assembly on chip. Nat. Commun. 7, 10447 (2016).
Deshpande, S., Birnie, A. & Dekker, C. On-chip density-based purification of liposomes. Biomicrofluidics 11, 34106 (2017).
Shum, H.C., Lee, D., Yoon, I., Kodger, T. & Weitz, D.A. Double emulsion templated monodisperse phospholipid vesicles. Langmuir 24, 7651–7653 (2008).
Teh, S.Y., Khnouf, R., Fan, H. & Lee, A.P. Stable, biocompatible lipid vesicle generation by solvent extraction-based droplet microfluidics. Biomicrofluidics 5, 44113 (2011).
Utada, A.S. et al. Monodisperse double emulsions generated from a microcapillary device. Science 308, 537–541 (2005).
Walde, P., Cosentino, K., Engel, H. & Stano, P. Giant vesicles: preparations and applications. ChemBioChem 11, 848–865 (2010).
Spoelstra, K.W., Deshpande, S. & Dekker, C. Tailoring the appearance: what will synthetic cells look like? Curr. Opin. Biotechnol. 51, 47–56 (2018).
Forster, A.C. & Church, G.M. Towards synthesis of a minimal cell. Mol. Syst. Biol. 2, 45 (2006).
Elani, Y. Construction of membrane-bound artificial cells using microfluidics: a new frontier in bottom-up synthetic biology. Biochem. Soc. Trans. 44, 723–730 (2016).
Caspi, Y. & Dekker, C. Divided we stand: splitting synthetic cells for their proliferation. Syst. Synth. Biol. 8, 249–269 (2014).
Szwedziak, P., Wang, Q., Bharat, T.A.M., Tsim, M. & Löwe, J. Architecture of the ring formed by the tubulin homologue FtsZ in bacterial cell division. Elife 3, e04601 (2014).
Cabré, E.J. et al. Bacterial division proteins FtsZ and ZipA induce vesicle shrinkage and cell membrane invagination. J. Biol. Chem. 288, 26625–26634 (2013).
Osawa, M. & Erickson, H.P. Liposome division by a simple bacterial division machinery. Proc. Natl. Acad. Sci. USA 110, 11000–11004 (2013).
Keber, F.C. et al. Topology and dynamics of active nematic vesicles. Science 345, 1135–1139 (2014).
Carvalho, K. et al. Cell-sized liposomes reveal how actomyosin cortical tension drives shape change. Proc. Natl. Acad. Sci. USA 110, 16456–16461 (2013).
Cáceres, R., Abou-Ghali, M. & Plastino, J. Reconstituting the actin cytoskeleton at or near surfaces in vitro. Biochim. Biophys. Acta 1853, 3006–3014 (2015).
van Nies, P. et al. Unbiased tracking of the progression of mRNA and protein synthesis in bulk and in liposome-confined reactions. ChemBioChem 14, 1963–1966 (2013).
Scott, A. et al. Cell-free phospholipid biosynthesis by gene-encoded enzymes reconstituted in liposomes. PLoS One 11, e0163058 (2016).
Noireaux, V. & Libchaber, A. A vesicle bioreactor as a step toward an artificial cell assembly. Proc. Natl. Acad. Sci. USA 101, 17669–17674 (2004).
Gardner, P.M., Winzer, K. & Davis, B.G. Sugar synthesis in a protocellular model leads to a cell signalling response in bacteria. Nat. Chem. 1, 377–383 (2009).
Lentini, R. et al. Two-way chemical communication between artificial and natural cells. ACS Cent. Sci. 3, 117–123 (2017).
Adamala, K.P., Martin-Alarcon, D.A., Guthrie-Honea, K.R. & Boyden, E.S. Engineering genetic circuit interactions within and between synthetic minimal cells. Nat. Chem. 9, 431–439 (2016).
Cama, J. et al. Direct optofluidic measurement of the lipid permeability of fluoroquinolones. Sci. Rep. 6, 32824 (2016).
Cama, J., Chimerel, C., Pagliara, S., Javer, A. & Keyser, U.F. A label-free microfluidic assay to quantitatively study antibiotic diffusion through lipid membranes. Lab Chip 14, 2303–8 (2014).
Dezi, M., Di Cicco, A., Bassereau, P. & Lévy, D. Detergent-mediated incorporation of transmembrane proteins in giant unilamellar vesicles with controlled physiological contents. Proc. Natl. Acad. Sci. USA 110, 7276–81 (2013).
Zhao, H. & Lappalainen, P. A simple guide to biochemical approaches for analyzing protein-lipid interactions. Mol. Biol. Cell 23, 2823–2830 (2012).
Lemière, J., Valentino, F., Campillo, C. & Sykes, C. How cellular membrane properties are affected by the actin cytoskeleton. Biochimie 130, 33–40 (2016).
Kahya, N. Protein-protein and protein-lipid interactions in domain-assembly: lessons from giant unilamellar vesicles. Biochim. Biophys. Acta 1798, 1392–1398 (2010).
Estes, D.J., Lopez, S.R., Fuller, A.O. & Mayer, M. Triggering and visualizing the aggregation and fusion of lipid membranes in microfluidic chambers. Biophys. J. 91, 233–43 (2006).
Terasawa, H., Nishimura, K., Suzuki, H., Matsuura, T. & Yomo, T. Coupling of the fusion and budding of giant phospholipid vesicles containing macromolecules. Proc. Natl. Acad. Sci. USA 109, 5942–5947 (2012).
Karatekin, E. et al. Cascades of transient pores in giant vesicles: line tension and transport. Biophys. J. 84, 1734–1749 (2003).
Oglęcka, K., Rangamani, P., Liedberg, B., Kraut, R.S. & Parikh, A.N. Oscillatory phase separation in giant lipid vesicles induced by transmembrane osmotic differentials. Elife 3, e03695 (2014).
Carugo, D., Bottaro, E., Owen, J., Stride, E. & Nastruzzi, C. Liposome production by microfluidics: potential and limiting factors. Sci. Rep. 6, 25876 (2016).
Zylberberg, C. & Matosevic, S. Pharmaceutical liposomal drug delivery: a review of new delivery systems and a look at the regulatory landscape. Drug Deliv. 23, 3319–3329 (2016).
Yang, J. et al. Drug delivery via cell membrane fusion using lipopeptide modified liposomes. ACS Cent. Sci. 2, 621–630 (2016).
Armada-Moreira, A., Taipaleenmäki, E., Itel, F., Zhang, Y. & Städler, B. Droplet-microfluidics towards the assembly of advanced building blocks in cell mimicry. Nanoscale 8, 19510–19522 (2016).
Edwards, K.A., Bolduc, O.R. & Baeumner, A.J. Miniaturized bioanalytical systems: enhanced performance through liposomes. Curr. Opin. Chem. Biol. 16, 444–452 (2012).
Jesorka, A. & Orwar, O. Liposomes: technologies and analytical applications. Annu. Rev. Anal. Chem. 1, 801–832 (2008).
Thiele, J. Polymer material design by microfluidics inspired by cell biology and cell-free biotechnology. Macromol. Chem. Phys. 218, 1600429 (2017).
Reeves, J.P. & Dowben, R.M. Formation and properties of thin-walled phospholipid vesicles. J. Cell. Physiol. 73, 49–60 (1969).
Olson, F., Hunt, C.A., Szoka, F.C., Vail, W.J. & Papahadjopoulos, D. Preparation of liposomes of defined size distribution by extrusion through polycarbonate membranes. Biochim. Biophys. Acta 557, 9–23 (1979).
Angelova, M.I. & Dimitrov, D.S. Liposome electroformation. Faraday Discuss. Chem. Soc. 81, 303 (1986).
Yu, B., Lee, R.J. & Lee, L.J. Microfluidic methods for production of liposomes. Methods Enzymol. 465, 129–141 (2009).
Deng, N.N., Yelleswarapu, M. & Huck, W.T.S. Monodisperse uni- and multicompartment liposomes. J. Am. Chem. Soc. 138, 7584–7591 (2016).
Mozafari, M.R. Liposomes: an overview of manufacturing techniques. Cell. Mol. Biol. Lett. 10, 711–719 (2005).
Lotter, J., Botes, A.L., Van Dyk, M.S. & Breytenbach, J.C. Correlation between the physicochemical properties of organic solvents and their biocompatibility toward epoxide hydrolase activity in whole-cells of a yeast, Rhodotorula sp.. Biotechnol. Lett. 26, 1191–1195 (2004).
Jiménez, M., Martos, A., Cabré, E.J., Raso, A. & Rivas, G. Giant vesicles: a powerful tool to reconstruct bacterial division assemblies in cell-like compartments. Environ. Microbiol. 15, 3158–3168 (2013).
Nunes, J.K., Tsai, S.S.H., Wan, J. & Stone, H.A. Dripping and jetting in microfluidic multiphase flows applied to particle and fibre synthesis. J. Phys. D Appl. Phys. 46, 114002 (2013).
Ho, C.S., Kim, J.W. & Weitz, D.A. Microfluidic fabrication of monodisperse biocompatible and biodegradable polymersomes with controlled permeability. J. Am. Chem. Soc. 130, 9543–9549 (2008).
Pautot, S., Frisken, B.J. & Weitz, D.A. Engineering asymmetric vesicles. Proc. Natl. Acad. Sci. USA 100, 10718–10721 (2003).
Richmond, D.L. et al. Forming giant vesicles with controlled membrane composition, asymmetry, and contents. Proc. Natl. Acad. Sci. USA 108, 9431–9436 (2011).
Matosevic, S. & Paegel, B.M. Layer-by-layer cell membrane assembly. Nat. Chem. 5, 958–63 (2013).
Knovel Critical Tables (2008) Available at: https://app.knovel.com/web/toc.v/cid:kpKCTE000X/viewerType:toc/root_slug:knovel-critical-tables (accessed 27th February, 2018).
Campillo, C. et al. Unexpected membrane dynamics unveiled by membrane nanotube extrusion. Biophys. J. 104, 1248–1256 (2013).
Surewicz, W.K. Membrane actions of water-soluble fusogens: effect of dimethyl sulfoxide, glycerol and sucrose on lipid bilayer order and fluidity. Chem. Phys. Lipids 34, 363–372 (1984).
Cheng, C.Y., Wang, J.Y., Kausik, R., Lee, K.Y.C. & Han, S. Nature of interactions between PEO-PPO-PEO triblock copolymers and lipid membranes: (II) role of hydration dynamics revealed by dynamic nuclear polarization. Biomacromolecules 13, 2624–2633 (2012).
Kazayama, Y., Teshima, T., Osaki, T., Takeuchi, S. & Toyota, T. Integrated microfluidic system for size-based selection and trapping of giant vesicles. Anal. Chem. 88, 1111–1116 (2016).
Akagi, J. et al. Miniaturized embryo array for automated trapping, immobilization and microperfusion of zebrafish embryos. PLoS One 7 (2012).
Nishimura, K., Suzuki, H., Toyota, T. & Yomo, T. Size control of giant unilamellar vesicles prepared from inverted emulsion droplets. J. Colloid Interface Sci. 376, 119–125 (2012).
Abkarian, M., Loiseau, E. & Massiera, G. Continuous droplet interface crossing encapsulation (cDICE) for high throughput monodisperse vesicle design. Soft Matter 7, 4610 (2011).
Jahn, A., Vreeland, W.N., Gaitan, M. & Locascio, L.E. Controlled vesicle self-assembly in microfluidic channels with hydrodynamic focusing. J. Am. Chem. Soc. 126, 2674–2675 (2004).
Jahn, A. et al. Microfluidic mixing and the formation of nanoscale lipid vesicles. ACS Nano 4, 2077–2087 (2010).
Stachowiak, J.C. et al. Unilamellar vesicle formation and encapsulation by microfluidic jetting. Proc. Natl. Acad. Sci. USA 105, 4697–4702 (2008).
Funakoshi, K., Suzuki, H. & Takeuchi, S. Formation of giant lipid vesiclelike compartments from a planar lipid membrane by a pulsed jet flow. J. Am. Chem. Soc. 129, 12608–12609 (2007).
Acknowledgements
We would like to acknowledge Y. Caspi, A. Birnie, F. Fanalista, K. Spoelstra, D. Hueting, M. van Doorn, M. Schaich, K. Al-Nahas, and S. Sachdev for their contributions to the development of the described protocol and valuable feedback. This work was supported by an NWO TOP-PUNT grant (no. 718014001), the Netherlands Organisation for Scientific Research (NWO/OCW) and a European Research Council Advanced Grant SynDiv (no. 669598).
Author information
Authors and Affiliations
Contributions
S.D. performed the experiments. S.D. and C.D. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Schematic flow diagram summarizing the key steps involved in the preparation of a master wafer and subsequent steps leading to the production of a microfluidic device.
(a) Master wafer is prepared in the clean room. A silicon wafer is spin-coated with a suitable negative resist and pre-baked. The wafer is then selectively exposed to e-beam to write the desired pattern. The wafer is further post-baked and chemically developed to remove the non-patterned resist. The developed wafer is appropriately etched, cleaned, and silanized to obtain a master wafer. (b) The microfluidic device is prepared in the wet lab. PDMS is poured over the master and cured by baking. The cured PDMs is then carefully peeled off and holes are punched at appropriate locations. The PDMS surface is then activated using plasma and bonded to a PDMS-coated, surface-activated glass slide. The bonded device is surface-treated to render it partially hydrophilic. The device is now ready to perform OLA. The master can be used multiple times.
Supplementary Figure 2 Various steps involved in the making of a microfluidic device.
(a) Master wafer enclosed within a well-like structure made up of aluminium foil. The design patterns are faintly visible on the wafer surface. (b) Cut-out PDMS block having a single OLA design (faint channels can be seen). Inlets, exit and separation hole are punched. (c) Glass slides (seen as faint outlines) immersed in a thin layer of PDMS, which is spread on a blank wafer. (d) Cured PDMS layer is peeled off to obtain PDMS-coated glass slides. The left-most glass slide is damaged severely during the peeling-off process and thus will not be used, while the down-most glass slide is broken only in the corner and can be used further. The remaining two glass slides are intact and in good condition. One glass slide has been removed showing a clean, reusable wafer surface. (e) A microfluidic device prepared by bonding the PDMS-coated glass slide to the design-containing PDMS block, right after the plasma treatment. The microfluidic channels are clearly visible. (f) Thicker (outer tube) and thinner (outer tube) tubes, placed in fittings, with the corresponding metal connectors inserted at one end.
Supplementary Figure 3 Production junction with bumpers.
Bright-field image of the production junction having bumper-like protrusions, in order to facilitate the surface treatment.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3. (PDF 579 kb)
Supplementary Data
.dxf file containing a standard design for OLA and subsequent liposome purification from 1-octanol droplets. (ZIP 91 kb)
Partial hydrophilic surface treatment of the microfluidic device.
1% (vol/vol) PVA solution is used. IA and LO channel (air) pressure: 50 mbar; OA channel (PVA) pressure: 120 mbar. (AVI 5817 kb)
Optimal double-emulsion droplet production.
IA is 15% (vol/vol) glycerol; LO is 0.2% (wt/vol) lipids (DOPC/Rh-PE in a molar ratio of 1,000:1) in 1-octanol; OA is 15% (vol/vol) glycerol and 5% (wt/vol) P188. (AVI 2960 kb)
Double-emulsion droplets with a prominent 1-octanol pocket flowing downstream.
IA is 15% (vol/vol) glycerol; LO is 0.2% (wt/vol) lipids (DOPC/Rh-PE in a molar ratio of 1,000:1) in 1-octanol; OA is 15% (vol/vol) glycerol and 5% (wt/vol) P188. (AVI 5173 kb)
Liposomes and budded-off 1-octanol droplets further downstream of the channel.
IA is 15% (vol/vol) glycerol; LO is 0.2% (wt/vol) lipids (DOPC/Rh-PE in a molar ratio of 1,000:1) in 1-octanol; OA is 15% (vol/vol) glycerol and 5% (wt/vol) P188. (AVI 1786 kb)
A top view across the separation hole, showing the separation process.
IA is 15% (vol/vol) glycerol; LO is 0.2% (wt/vol) lipids (DOPC/Rh-PE in a molar ratio of 1,000:1) in 1-octanol; OA is 15% (vol/vol) glycerol and 5% (wt/vol) P188. (AVI 9768 kb)
Optimal separation process, mainly yielding liposomes into the post-hole channel.
IA is 15% (vol/vol) glycerol; LO is 0.2% (wt/vol) lipids (DOPC/Rh-PE in a molar ratio of 1,000:1) in 1-octanol; OA is 15% (vol/vol) glycerol and 5% (wt/vol) P188. (AVI 383 kb)
Suboptimal production, yet double-emulsion droplets are still formed.
IA is 15% (vol/vol) glycerol; LO is 0.2% (wt/vol) lipids (DOPC/Rh-PE in a molar ratio of 1,000:1) in 1-octanol; OA is 15% (vol/vol) glycerol and 5% (wt/vol) P188. (AVI 9372 kb)
Suboptimal post-junction channel, showing aggregates of liposomes and 1-octanol droplets.
IA is 15% (vol/vol) glycerol; LO is 0.2% (wt/vol) lipids (DOPC/Rh-PE in a molar ratio of 1,000:1) in 1-octanol; OA is 15% (vol/vol) glycerol and 5% (wt/vol) P188. (AVI 474 kb)
Suboptimal purification process, showing unwanted 1-octanol aggregates entering the post-hole channel.
IA is 15% (vol/vol) glycerol; LO is 0.2% (wt/vol) lipids (DOPC/Rh-PE in a molar ratio of 1,000:1) in 1-octanol; OA is 15% (vol/vol) glycerol and 5% (wt/vol) P188. (AVI 434 kb)
Rights and permissions
About this article
Cite this article
Deshpande, S., Dekker, C. On-chip microfluidic production of cell-sized liposomes. Nat Protoc 13, 856–874 (2018). https://doi.org/10.1038/nprot.2017.160
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2017.160
This article is cited by
-
A simple method to make, trap and deform a vesicle in a gel
Scientific Reports (2023)
-
An ultrasensitive microfluidic approach reveals correlations between the physico-chemical and biological activity of experimental peptide antibiotics
Scientific Reports (2022)
-
High-resolution imaging of bacterial spatial organization with vertical cell imaging by nanostructured immobilization (VerCINI)
Nature Protocols (2022)
-
Building programmable multicompartment artificial cells incorporating remotely activated protein channels using microfluidics and acoustic levitation
Nature Communications (2022)
-
Investigation of the programmed cell death by encapsulated cytoskeleton drug liposomes using a microfluidic platform
Microfluidics and Nanofluidics (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.