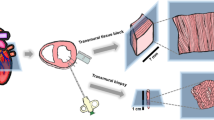
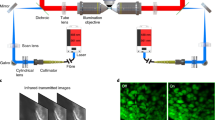
Abstract
This protocol describes the preparation of highly viable adult ventricular myocardial slices from the hearts of small and large mammals, including rodents, pigs, dogs and humans. Adult ventricular myocardial slices are 100- to 400-μm-thick slices of living myocardium that retain the native multicellularity, architecture and physiology of the heart. This protocol provides a list of the equipment and reagents required alongside a detailed description of the methodology for heart explantation, tissue preparation, slicing with a vibratome and handling of myocardial slices. Supplementary videos are included to visually demonstrate these steps. A number of critical steps are addressed that must be followed in order to prepare highly viable myocardial slices. These include identification of myocardial fiber direction and fiber alignment within the tissue block, careful temperature control, use of an excitation–contraction uncoupler, optimal vibratome settings and correct handling of myocardial slices. Many aspects of cardiac structure and function can be studied using myocardial slices in vitro. Typical results obtained with hearts from a small mammal (rat) and a large mammal (human) with heart failure are shown, demonstrating myocardial slice viability, maximum contractility, Ca2+ handling and structure. This protocol can be completed in ∼4 h.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
References
De Boer, T.P., Camelliti, P., Ravens, U. & Kohl, P. Myocardial tissue slices: organotypic pseudo-2D models for cardiac research & development. Future Cardiol. 5, 425–430 (2009).
Brandenburger, M. et al. Organotypic slice culture from human adult ventricular myocardium. Cardiovasc. Res. 93, 50–59 (2012).
Perbellini, F. et al. Investigation of cardiac fibroblasts using myocardial slices. Cardiovasc. Res. 119, 909–920 (2017).
Neri, B., Cini-Neri, G. & D'Alterio, M. Effect of anthracyclines and mitoxantrone on oxygen uptake and ATP intracellular concentration in rat heart slices. Biochem. Biophys. Res. Commun. 125, 954–960 (1984).
Wang, K. et al. Cardiac tissue slices: preparation, handling, and successful optical mapping. Am. J. Physiol. Heart Circ. Physiol. 308, H1112–H1125 (2015).
Camelliti, P. et al. Adult human heart slices are a multicellular system suitable for electrophysiological and pharmacological studies. J. Mol. Cell Cardiol. 51, 390–398 (2011).
Kang, C. et al. Human organotypic cultured cardiac slices: new platform for high throughput preclinical human trials. Sci. Rep. 6, 28798 (2016).
Claycomb, W.C. Biochemical aspects of cardiac muscle differentiation. Possible control of deoxyribonucleic acid synthesis and cell differentiation by adrenergic innervation and cyclic adenosine 3′:5′-monophosphate. J. Biol. Chem. 251, 6082–6089 (1976).
Yasuhara, S., Takaki, M., Kikuta, A., Ito, H. & Suga, H. Myocardial VO2 of mechanically unloaded contraction of rat ventricular slices measured by a new approach. Am. J. Physiol. 270 (3 Part 2): H1063–H1070 (1996).
Burnashev, N.A., Edwards, F.A. & Verkhratsky, A.N. Patch-clamp recordings on rat cardiac muscle slices. Pflugers Arch. 417, 123–125 (1990).
Katano, Y., Akera, T., Temma, K. & Kennedy, R.H. Enhanced ouabain sensitivity of the heart and myocardial sodium pump in aged rats. Eur. J. Pharmacol. 105, 95–103 (1984).
Bursac, N. et al. Cultivation in rotating bioreactors promotes maintenance of cardiac myocyte electrophysiology and molecular properties. Tissue Eng. 9, 1243–1253 (2003).
Bussek, A. et al. Tissue slices from adult mammalian hearts as a model for pharmacological drug testing. Cell Physiol. Biochem. 24, 527–536 (2015).
Bussek, A. et al. Cardiac tissue slices with prolonged survival for in vitro drug safety screening. J. Pharmacol. Toxicol. Methods 66, 145–151 (2012).
Pillekamp, F. et al. Neonatal murine heart slices. A robust model to study ventricular isometric contractions. Cell Physiol. Biochem. 20, 837–846 (2007).
Hannes, T. et al. Biological pacemakers: characterization in an in vitro coculture model. J. Electrocardiol. 41, 562–566 (2008).
Mawad, D. et al. A conducting polymer with enhanced electronic stability applied in cardiac models. Sci. Adv. 2, e1601007 (2016).
Takaki, M. et al. Sarcoplasmic reticulum Ca2+ pump blockade decreases O2 use of unloaded contracting rat heart slices: thapsigargin and cyclopiazonic acid. J. Mol. Cell Cardiol. 30, 649–659 (1998).
Kohzuki, H. et al. Energy expenditure by Ba(2+) contracture in rat ventricular slices derives from cross-bridge cycling. Am. J. Physiol. 277 (1 Partt 2): H74–H79 (1999).
Kohzuki, H., Misawa, H., Sakata, S., Ohga, Y. & Takaki, M. Sustained high O2 use for Ca2+ handling in rat ventricular slices under decreased free shortening after ryanodine. Am. J. Physiol. Heart Circ. Physiol. 281, H566–H572 (2001).
Yamashita, D. et al. O2 consumption of mechanically unloaded contractions of mouse left ventricular myocardial slices. AJP Hear Circ. Physiol. 287, H54–H62 (2004).
Hattori, H. et al. NHE-1 blockade reversed changes in calcium transient in myocardial slices from isoproterenol-induced hypertrophied rat left ventricle. Biochem. Biophys. Res. Commun. 419, 431–435 (2012).
Takeshita, D. et al. Effects of formaldehyde on cardiovascular system in in situ rat hearts. Basic Clin. Pharmacol. Toxicol. 105, 271–280 (2009).
Shimizu, J. et al. Increased O2 consumption in excitation–contraction coupling in hypertrophied rat heart slices related to increased Na+?Ca2+ exchange activity. J. Physiol. Sci. 59, 63–74 (2009).
Perbellini, F. et al. Free-of-acrylamide SDS-based tissue clearing (FASTClear) for three dimensional visualization of myocardial tissue. Sci. Rep. 7, 5188 (2017).
Pillekamp, F. et al. Force measurements of human embryonic stem cell-derived cardiomyocytes in an in vitro transplantation model. Stem Cells 25, 174–180 (2007).
Habeler, W., Peschanski, M. & Monville, C. Organotypic heart slices for cell transplantation and physiological studies. Organogenesis 5, 62–66 (2009).
Cartledge, J.E. et al. Functional crosstalk between cardiac fibroblasts and adult cardiomyocytes by soluble mediators. Cardiovasc. Res. 105, 260–270 (2015).
Civitarese, R.A., Kapus, A., McCulloch, C.A. & Connelly, K.A. Role of integrins in mediating cardiac fibroblast–cardiomyocyte cross talk: a dynamic relationship in cardiac biology and pathophysiology. Basic Res. Cardiol. 112, 6 (2017).
Louch, W.E., Sheehan, K.A. & Wolska, B.M. Methods in cardiomyocyte isolation, culture, and gene transfer. J. Mol. Cell Cardiol. 51, 288–298 (2011).
Zhang, Y. et al. Dedifferentiation and proliferation of mammalian cardiomyocytes. PLoS One 5, e12559 (2010).
Mitcheson, J.S., Hancox, J.C. & Levi, A.J. Action potentials, ion channel currents and transverse tubule density in adult rabbit ventricular myocytes maintained for 6 days in cell culture. Pflugers Arch. 431, 814–827 (1996).
Shattock, M.J., Bullock, G., Manning, A.S., Young, M. & Hearse, D.J. Limitations of the isolated papillary muscle as an experimental model: a metabolic, functional and ultrastructural study. Clin. Sci. 64 (2) 4P (1983).
Malan, D. et al. Human iPS cell model of type 3 long QT syndrome recapitulates drug-based phenotype correction. Basic Res. Cardiol. 111, 14 (2016).
Robertson, C., Tran, D.D. & George, S.C. Concise review: maturation phases of human pluripotent stem cell-derived cardiomyocytes. Stem Cells 31, 829–837 (2013).
Wu, J., Ocampo, A. & Belmonte, J. Cellular metabolism and induced pluripotency. Cell 166, 1371–1385 (2016).
Kane, C. & Terracciano, C.M. Induced pluripotent stem cell-derived cardiac myocytes to understand and test calcium handling: pie in the sky? J. Mol. Cell. Cardiol. 89, 376–378 (2015).
Frieler, R.A. & Mortensen, R.M. Immune cell and other noncardiomyocyte regulation of cardiac hypertrophy and remodeling. Circulation 131, 1019–1030 (2015).
Cordeiro, J.M., Greene, L., Heilmann, C., Antzelevitch, D. & Antzelevitch, C. Transmural heterogeneity of calcium activity and mechanical function in the canine left ventricle. Am. J. Physiol. 286, H1471–H1479 (2004).
Antzelevitch, C. et al. Heterogeneity within the ventricular wall. Electrophysiology and pharmacology of epicardial, endocardial, and M cells. Circ Res. 69, 1427–1449 (1991).
Bigelow, W.G., Lindsay, W.K. & Greenwood, W.F. Hypothermia; its possible role in cardiac surgery: an investigation of factors governing survival in dogs at low body temperatures. Ann. Surg. 132, 849–866. (1950).
Dae, M.W., Gao, D.W., Sessler, D.I., Chair, K. & Stillson, C.A. Effect of endovascular cooling on myocardial temperature, infarct size, and cardiac output in human-sized pigs. Am. J. Physiol. 282, H1584–H1591 (2002).
Tissier, R., Ghaleh, B., Cohen, M.V., Downey, J.M. & Berdeaux, A. Myocardial protection with mild hypothermia. Cardiovasc. Res. 94, 217–225 (2012).
Azar, T., Sharp, J. & Lawson, D. Heart rates of male and female Sprague-Dawley and spontaneously hypertensive rats housed singly or in groups. J. Am. Assoc. Lab. Anim. Sci. 50, 175–184 (2011).
Bensley, J.G., De Matteo, R., Harding, R., Black, M.J. & Schwalb, H. Three-dimensional direct measurement of cardiomyocyte volume, nuclearity, and ploidy in thick histological sections. Sci. Rep. 6, 23756 (2016).
Acknowledgements
We thank the British Heart Foundation for funding our work, particularly the BHF Centre for Regenerative Medicine award at Imperial College London (RM/13/1/30157) and the MBBS PhD studentship to S.A.W. (FS/15/35/31529). We thank The Facility for Imaging by Light Microscopy (FILM) at Imperial College London, in particular S.M. Rothery. Human samples were provided by the NIHR Cardiovascular Biomedical Research Unit at the Royal Brompton and Harefield NHS Foundation Trust and Imperial College London. Canine samples were provided by GlaxoSmithKline. Porcine samples were provided by the Translational Biomedical Research Centre, University of Bristol.
Author information
Authors and Affiliations
Contributions
S.A.W. wrote the manuscript, collected data and contributed to the optimization of the protocol. M.S. collected data and contributed to the optimization of the protocol. I.B. contributed to the optimization of the protocol. R.A. provided porcine specimens. C.M.T. contributed to the optimization of the protocol. F.P. collected data and contributed to the optimization of the protocol. All authors proof-read the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Original vs. optimized protocol for rat (small mammal) & human HF (large mammal) myocardial slices.
Comparison between original and optimized protocols. Original protocol was based on several methods from previously published manuscripts(2,6,13). A) Rat myocardial slices produced using optimized protocol had a significantly higher maximum contractility compared to those produced with original protocol (0.39±0.05mN/mm2 vs. 11.55±1.30mN/mm2, Original: N=24/24, Optimized: N=10/10, Unpaired t-test). B) Human HF myocardial slices produced using optimized protocol had a significantly higher maximum contractility compared to those produced with original protocol (3.44±0.38mN/mm2 vs. 13.77±3.52mN/mm2, Original: N=20/20, Optimized: N=9/9, Unpaired t-test).
Imperial College London provided permission for the use of the animals in this study. All procedures were performed under license by the UK Home Office, in accordance with the United Kingdom Animals (Scientific Procedures) Act 1986. Animals were killed following guidelines established by the European Directive on the protection of animals used for scientific purposes (2010/63/EU).
Data is presented as mean ± standard error
N = number of slices/number of hearts
Supplementary information
Supplementary Text and Figures
Supplementary Figure 1. (PDF 183 kb)
Preparation of left ventricular tissue block from small mammalian heart (Rat) – Part 1 (video depicts Step 2A(i–vii)).
The preparation of the tissue block in this video has been carried out slowly to aid the visualization of the technique. However, tissue block preparation should be carried out as quickly as possible. Tissue should be kept cool throughout. If tissue contracts during block preparation, transfer to holding bath and allow to cool for 30 s. (MP4 24480 kb)
Preparation of left ventricular tissue block from small mammalian heart (Rat) – Part 2 (video depicts Step 2A(viii–x)).
The preparation of the tissue block in this video has been carried out slowly to aid the visualization of the technique. However, tissue block preparation should be carried out as quickly as possible. Tissue should be kept cool throughout. If tissue contracts during block preparation, transfer to holding bath and allow to cool for 30 s. (MP4 24867 kb)
Preparation of left ventricular tissue block from small mammalian heart (Rat) – Part 3 (video depicts Step 2A(xi).
The preparation of the tissue block in this video has been carried out slowly to aid the visualization of the technique. However, tissue block preparation should be carried out as quickly as possible. Tissue should be kept cool throughout. If tissue contracts during block preparation, transfer to holding bath and allow to cool for 30 s. (MP4 24584 kb)
Mounting of left ventricular tissue block on agarose-coated specimen holder.
Video depicts how to handle, dry and mount tissue block correctly. (MP4 18702 kb)
Slicing of left ventricular tissue block.
Video depicts bringing blade to 'starting position' and various stages of slicing. (MP4 26143 kb)
Handling and storage of myocardial slices.
Video depicts how to move a myocardial slice from a vibratome bath to a holding bath. Video also shows how myocardial slices should be kept in a holding bath. (MP4 9422 kb)
Culturing myocardial slices using air–liquid interface method.
Video depicts how to culture myocardial slices on an air–liquid interface using Transwell membranes as described by Brandenburger et al. (ref. 2). (MP4 24327 kb)
Myocardial slice contraction.
Video depicts a human HF myocardial slice contracting (field stimulation, 0.5 Hz, 30 V) and a rat myocardial slice contracting (point stimulation, 1 Hz, 10 V). (MP4 5001 kb)
Ca2+ handling of myocardial slices.
Rat myocardial slice was loaded with Fluo-4 AM (as described in 'Anticipated Results—Ca2+ handling'). Myocardial slice was field stimulated at 1 Hz, 10 V. Cardiomyocytes at the slice surface flash with each calcium transient. (MP4 9282 kb)
Rights and permissions
About this article
Cite this article
Watson, S., Scigliano, M., Bardi, I. et al. Preparation of viable adult ventricular myocardial slices from large and small mammals. Nat Protoc 12, 2623–2639 (2017). https://doi.org/10.1038/nprot.2017.139
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2017.139
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.