Abstract
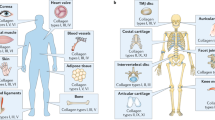
Collagen type I is the most abundant extracellular matrix protein, and collagen type I supramolecular assemblies (e.g., tissue grafts, biomaterials and cell-assembled systems) are used extensively in tissue engineering and regenerative medicine. Many studies, for convenience or economic reasons, do not accurately determine collagen type I purity, concentration, solubility and extent of cross-linking in biological specimens, frequently resulting in erroneous conclusions. In this protocol, we describe solubility; normal, reduced and delayed (interrupted) SDS-PAGE; hydroxyproline; Sircol collagen and Pierce BCA protein; denaturation temperature; ninhydrin/trinitrobenzene sulfonic acid; and collagenase assays and assess them in a diverse range of biological samples (e.g., tissue samples; purified solutions or lyophilized materials; 3D scaffolds, such as sponges and hydrogels; and cell media and layers). Collectively, the described protocols provide a comprehensive, yet fast and readily implemented, toolbox for collagen type I characterization in any biological specimen.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hulmes, D. Building collagen molecules, fibrils, and suprafibrillar structures. J. Struct. Biol. 137, 2–10 (2002).
Kielty, C. & Grant, M. The collagen family: structure, assembly, and organization in the extracellular matrix. in Connective Tissue and Its Heritable Disorders: Molecular, Genetic, and Medical Aspects (eds. Royce, P. & Steinmann, B.) 159–221 (Wiley, 2002).
Bella, J. Collagen structure: new tricks from a very old dog. Biochem. J. 473, 1001–1025 (2016).
Brodsky, B. & Persikov, A. Molecular structure of the collagen triple helix. Adv. Protein Chem. 70, 301–339 (2005).
Friess, W. Collagen – biomaterial for drug delivery. Eur. J. Pharm. Biopharm. 45, 113–136 (1998).
Glowacki, J. & Mizuno, S. Collagen scaffolds for tissue engineering. Biopolymers 89, 338–344 (2008).
Pawelec, K., Best, S. & Cameron, R. Collagen: a network for regenerative medicine. J. Mater. Chem. B 4, 6484–6496 (2016).
Ramshaw, J. Biomedical applications of collagens. J. Biomed. Mater. Res. B 104, 665–675 (2016).
Zeugolis, D., Paul, R. & Attenburrow, G. Factors influencing the properties of reconstituted collagen fibers prior to self-assembly: animal species and collagen extraction method. J. Biomed. Mater. Res. A 86A, 892–904 (2008).
Skierka, E. & Sadowska, M. The influence of different acids and pepsin on the extractability of collagen from the skin of Baltic cod (Gadus morhua). Food Chem. 105, 1302–1306 (2007).
Nalinanon, S., Benjakul, S., Visessanguan, W. & Kishimura, H. Use of pepsin for collagen extraction from the skin of bigeye snapper (Priacanthus tayenus). Food Chem. 104, 593–601 (2007).
Lynn, A., Yannas, I. & Bonfield, W. Antigenicity and immunogenicity of collagen. J. Biomed. Mater. Res. B 71B, 343–354 (2004).
Gelman, R., Poppke, D. & Piez, K. Collagen fibril formation in vitro. The role of the nonhelical terminal regions. J. Biol. Chem. 254, 11741–11745 (1979).
Pontz, B., Meigel, W., Rauterberg, J. & Kühn, K. Localization of two species specific antigenic determinants on the peptide chains of calf skin collagen. Eur. J. Biochem. 16, 50–54 (1970).
Zeugolis, D. et al. Collagen solubility testing, a quality assurance step for reproducible electro-spun nano-fibre fabrication. A technical note. J. Biomater. Sci. Polym. Ed. 19, 1307–1317 (2008).
Browne, S., Zeugolis, D. & Pandit, A. Collagen: finding a solution for the source. Tissue Eng. A 19, 1491–1494 (2013).
Peng, Y., Glattauer, V., Werkmeister, J. & Ramshaw, J. Evaluation for collagen products for cosmetic application. J. Cosmet. Sci. 55, 327–341 (2004).
Picha, B., Thompson, M. & Vondriska, T. Preclinical trials: keep 'reproducibility' in context. Nature 485, 41 (2012).
Pusztai, L., Hatzis, C. & Andre, F. Reproducibility of research and preclinical validation: problems and solutions. Nat. Rev. Clin. Oncol. 10, 720–724 (2013).
Begley, C. & Ioannidis, J. Reproducibility in science: improving the standard for basic and preclinical research. Circ. Res. 116, 116–126 (2015).
Coller, B. & Califf, R. Traversing the valley of death: a guide to assessing prospects for translational success. Sci. Transl. Med. 1, 10cm19 (2009).
Fitch, S., Harkness, M. & Harkness, R. Extraction of collagen from tissues. Nature 176, 163 (1955).
Gross, J., Highberger, J. & Schmitt, F. Extraction of collagen from connective tissue by neutral salt solutions. Proc. Natl. Acad. Sci. USA 41, 1–7 (1955).
Bakerman, S. Quantitative extraction of acid-soluble human skin collagen with age. Nature 196, 375–376 (1962).
Miller, E. & Rhodes, R. Preparation and characterization of the different types of collagen. Methods Enzymol. 82, 33–64 (1982).
Rajan, N. et al. Preparation of ready-to-use, storable and reconstituted type I collagen from rat tail tendon for tissue engineering applications. Nat. Protoc. 1, 2753–2758 (2006).
Laemmli, U. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 (1970).
Sykes, B., Puddle, B., Francis, M. & Smith, R. The estimation of two collagens from human dermis by interrupted gel electrophoresis. Biochem. Biophys. Res. Commun. 72, 1472–1480 (1976).
Switzer, R., Merril, C. & Shifrin, S. A highly sensitive silver stain for detecting proteins and peptides in polyacrylamide gels. Anal. Biochem. 98, 231–237 (1979).
Udenfriend, S. Formation of hydroxyproline in collagen. Science 152, 1335–1340 (1966).
Barnes, M., Constable, B. & Kodicek, E. Studies in vivo on the biosynthesis of collagen and elastin in ascorbic acid-deficient guinea pigs. Biochem. J. 113, 387–397 (1969).
Blumenkrantz, N. & Asboe-Hansen, G. Biosynthesis of collagen and elastin by chick embryo skin during maturation. Arch. Dermatol. Forsch. 249, 99–104 (1974).
Faris, B. et al. The synthesis of connective tissue protein in smooth muscle cells. Biochim. Biophys. Acta 418, 93–103 (1976).
Spencer, H., Morgulis, S. & Wilder, V. A micromethod for the determination of gelatin and a study of the collagen content of muscles from normal and dystrophic rabbits. J. Biol. Chem. 120, 257–266 (1937).
Lightfoot, L. & Coolidge, T. The distribution of collagen in the guinea pig. J. Biol. Chem. 176, 477–484 (1948).
Lowry, O., Gilligan, D. & Katersky, E. The determination of collagen and elastin in tissues, with results obtained in various normal tissues from different species. J. Biol. Chem. 139, 795–804 (1941).
Neuman, R. & Logan, M. The determination of collagen and elastin in tissues. J. Biol. Chem. 186, 549–556 (1950).
Levine, R. A nanogram method for hydroxyproline. Mikrochim. Acta 5, 797–800 (1973).
Kessler, A., Rosen, H. & Levenson, S. Chromatographic fractionation of rat tail tendon collagen. Nature 184, 1640 (1959).
Kessler, A., Rosen, H. & Levenson, S. Chromatographic fractionation of acetic acid-solubilized rat tail tendon collagen. J. Biol. Chem. 235, 989–994 (1960).
Rucklidge, G. et al. Turnover rates of different collagen types measured by isotope ratio mass spectrometry. Biochim. Biophys. Acta 1156, 57–61 (1992).
Colgrave, M., Allingham, P. & Jones, A. Hydroxyproline quantification for the estimation of collagen in tissue using multiple reaction monitoring mass spectrometry. J. Chromatogr. A 1212, 150–153 (2008).
Böhlen, P. & Mellet, M. Automated fluorometric amino acid analysis: the determination of proline and hydroxyproline. Anal. Biochem. 94, 313–321 (1979).
Osborne, R., Longton, R. & Lamberts, B. Rapid improved amino acid analysis of collagen. Anal. Biochem. 44, 317–321 (1971).
Rydziel, S. & Canalis, E. Analysis of hydroxyproline by high performance liquid chromatography and its application to collagen turnover studies in bone cultures. Calcif. Tissue Int. 44, 421–424 (1989).
Green, G. & Reagan, K. Determination of hydroxyproline by high pressure liquid chromatography. Anal. Biochem. 201, 265–269 (1992).
Chan, S., Greaves, J., Da Silva, N. & Wang, S. Assaying proline hydroxylation in recombinant collagen variants by liquid chromatography-mass spectrometry. BMC Biotechnol. 12, 51 (2012).
Qiu, B. et al. Measurement of hydroxyproline in collagen with three different methods. Mol. Med. Rep. 10, 1157–1163 (2014).
Tredget, E. et al. Gas chromatography-mass spectrometry determination of 18O2 in 18O-labelled 4-hydroxyproline for measurement of collagen synthesis and intracellular degradation. J. Chromatogr. 612, 7–19 (1993).
Meyer, M. & Morgenstern, B. Characterization of gelatine and acid soluble collagen by size exclusion chromatography coupled with multi angle light scattering (SEC-MALS). Biomacromolecules 4, 1727–1732 (2003).
LeRoy, E., Harris, E. & Sjoerdsma, A. A modified procedure for radioactive hydroxyproline assay in urine and tissues after labeled proline administration. Anal. Biochem. 17, 377–382 (1966).
Gosslau, B. & Barrach, H. Enzyme-linked immunosorbent microassay for quantification of specific antibodies to collagen type I, II, III. J. Immunol. Methods 29, 71–77 (1979).
Laurent, G. Dynamic state of collagen: pathways of collagen degradation in vivo and their possible role in regulation of collagen mass. Am. J. Physiol. 252, 1–9 (1987).
Lareu, R. et al. Essential modification of the Sircol collagen assay for the accurate quantification of collagen content in complex protein solutions. Acta Biomater. 6, 3146–3151 (2010).
Gross, J. Thermal denaturation of collagen in the dispersed and solid state. Science 143, 960–961 (1964).
Rao, S. et al. A versatile microassay for elastase using succinylated elastin. Anal. Biochem. 250, 222–227 (1997).
Blumenkrantz, N. Automated triple assay for proline, hydroxyproline and hydroxylysine on one single sample. Clin. Biochem. 13, 177–183 (1980).
Ward, J. et al. Amine functionalization of collagen matrices with multifunctional polyethylene glycol systems. Biomacromolecules 11, 3093–3101 (2010).
Mathrubutham, M. & Rao, S. Single microassay for matrix degrading enzymes. Front. Biosci. 6, 13–16 (2001).
Kohn, R. & Rollerson, E. Aging of human collagen in relation to susceptibility to the action of collagenase. J. Gerontol. 15, 10–14 (1960).
Nagai, Y. Collagenase digestion of collagen. J. Biochem. 50, 486–492 (1961).
Zeugolis, D. & Raghunath, M. The physiological relevance of wet versus dry differential scanning calorimetry for biomaterial evaluation: a technical note. Polym. Int. 59, 1403–1407 (2010).
Graham, L. & Mechanic, G. Simultaneous determination of the reducible and nonreducible cross-links of connective tissue. Analysis of mineralized and nonmineralized bone collagen. Biochemistry 28, 7889–7895 (1989).
Pearson, C., Ainsworth, L. & Chovelon, A. The determination of small amounts of collagen hydroxylysyl glycosides. Connect. Tissue Res. 6, 51–59 (1978).
Helling, A. et al. In vitro enzymatic degradation of tissue graftsand collagen biomaterials by matrix metalloproteinases: improving the collagenase assay. ACS Biomater. Sci. Eng. 3, 1922–1932 (2017).
Bentley, J. & Hanson, A. The hydroxyproline of elastin. Biochim. Biophys. Acta 175, 339–344 (1969).
Dietz, A., Lubrano, T., Covault, H. & Rubinstein, H. Correct for hydroxyproline in elastin when measuring collagen in tissues with a high elastin content. Clin. Chem. 28, 1709 (1982).
Veis, A., Anesay, J. & Cohen, J. The long range reorganization of gelatin to the collagen structure. Arch. Biochem. Biophys. 94, 20–31 (1961).
Danielsen, C. Thermal stability of reconstituted collagen fibrils. Shrinkage characteristics upon in vitro maturation. Mech. Ageing Dev. 15, 269–278 (1981).
Hörmann, H. & Schlebusch, H. Reversible and irreversible denaturation of collagen fibers. Biochemistry 10, 932–937 (1971).
Kopp, J., Bonnet, M. & Renou, J. Effect of collagen crosslinking on collagen-water interactions (a DSC investigation). Matrix 9, 443–450 (1989).
Chen, R., Ho, H. & Sheu, M. Characterization of collagen matrices crosslinked using microbial transglutaminase. Biomaterials 26, 4229–4235 (2005).
Li, M. et al. Electrospinning polyaniline-contained gelatin nanofibers for tissue engineering applications. Biomaterials 27, 2705–2715 (2006).
Zeugolis, D., Paul, G. & Attenburrow, G. Cross-linking of extruded collagen fibers – a biomimetic three-dimensional scaffold for tissue engineering applications. J. Biomed. Mater. Res. A 89, 895–908 (2009).
Satyam, A. et al. In vitro evaluation of Ficoll-enriched and genipin-stabilised collagen scaffolds. J. Tissue Eng. Regen. Med. 8, 233–241 (2014).
Buttafoco, L. et al. Electrospinning of collagen and elastin for tissue engineering applications. Biomaterials 27, 724–734 (2006).
Bigi, A. et al. Mechanical and thermal properties of gelatin films at different degrees of glutaraldehyde crosslinking. Biomaterials 22, 763–768 (2001).
Kumar, P. et al. Low oxygen tension and macromolecular crowding accelerate extracellular matrix deposition in human corneal fibroblast culture. J. Tissue Eng. Regen. Med. (in the press).
Kumar, P. et al. Macromolecularly crowded in vitro microenvironments accelerate the production of extracellular matrix-rich supramolecular assemblies. Sci. Rep. 5, 8729 (2015).
Kumar, P. et al. Accelerated development of supramolecular corneal stromal-like assemblies from corneal fibroblasts in the presence of macromolecular crowders. Tissue Eng. C Methods 21, 660–670 (2015).
Satyam, A. et al. Low, but not too low, oxygen tension and macromolecular crowding accelerate extracellular matrix deposition in human dermal fibroblast culture. Acta Biomater. 44, 221–231 (2016).
Satyam, A. et al. Macromolecular crowding meets tissue engineering by self-assembly: a paradigm shift in regenerative medicine. Adv. Mater. 26, 3024–3034 (2014).
Cigognini, D. et al. Macromolecular crowding meets oxygen tension in human mesenchymal stem cell culture — a step closer to physiologically relevant in vitro organogenesis. Sci. Rep. 6, 30746 (2016).
Zeugolis, D. et al. An in situ and in vitro investigation for the transglutaminase potential in tissue engineering. J. Biomed. Mater. Res. A 92, 1310–1320 (2010).
English, A. et al. Substrate topography: a valuable in vitro tool, but a clinical red herring for in vivo tenogenesis. Acta Biomater. 27, 3–12 (2015).
Collin, E. et al. An injectable vehicle for nucleus pulposus cell-based therapy. Biomaterials 32, 1862–2870 (2011).
Abbah, S. et al. Co-transfection of decorin and interleukin-10 modulates pro-fibrotic extracellular matrix gene expression in human tenocyte culture. Sci. Rep. 6, 20922 (2016).
Tsekoura, E. et al. Battling bacterial infection with hexamethylene diisocyanate cross-linked and Cefaclor-loaded collagen scaffolds. Biomed. Mater. 12, 035013 (2017).
Sanami, M. et al. The influence of poly(ethylene glycol) ether tetrasuccinimidyl glutarate on the structural, physical, and biological properties of collagen fibers. J. Biomed. Mater. Res. B 104, 914–922 (2016).
Delgado, L., Fuller, K. & Zeugolis, D. Collagen cross-linking: biophysical, biochemical, and biological response analysis. Tissue Eng. A 23, 1064–1077 (2017).
Friess, W. & Lee, G. Basic thermoanalytical studies of insoluble collagen matrices. Biomaterials 17, 2289–2294 (1996).
Orban, J. et al. Crosslinking of collagen gels by transglutaminase. J. Biomed. Mater. Res. A 68, 756–762 (2004).
Miles, C. & Burjanadze, T. Thermal stability of collagen fibers in ethylene glycol. Biophys. J. 80, 1480–1486 (2001).
Acknowledgements
This work was supported by the Health Research Board, Health Research Awards Programme (grant agreement no. HRA_POR/2011/84); a Science Foundation Ireland, Career Development Award (grant agreement no. 15/CDA/3629); the Science Foundation Ireland/European Regional Development Fund (grant agreement no. 13/RC/2073); and the H2020, Marie Skłodowska-Curie Actions, Innovative Training Networks 2015 Tendon Therapy Train Project (grant agreement no. 676338). This work was also part of the Teagasc Walsh Fellowship (grant agreement no. 2014045) and the ReValueProtein Research Project (grant agreement no. 11/F/043) and was supported by the Department of Agriculture, Food and the Marine (DAFM) under the National Development Plan 2007–2013, funded by the Irish Government.
Author information
Authors and Affiliations
Contributions
H.C.-M., J.Q.C., V.G. and Z.W. contributed equally to this work and are listed in alphabetical order. H.C.-M., J.Q.C., V.G. and Z.W. designed and conducted the experiments and analyzed the data. D.I.Z. designed and supervised the study. All authors wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 SDS-PAGE and densitometric analysis of cell-synthesized collagen.
SDS-PAGE (a) and complementary densitometric analysis (b) of Symatese bovine skin collagen type I (1.6 μg/well) standard (A); acid-extracted collagen from WS1 fibroblast cell-layers (B, C); and pepsin-extracted collagen from WS1 fibroblast cell-layers (D, E). WS1 cells were cultured for 2 days in DMEM supplemented with 0.5 % FBS, 100 μg/ml of L-ascorbic acid 2-phosphate sesquimagnesium salt hydrate with (B, D) or without (C, E) 100 μg/ml of carrageenan (CR). The collagen was extracted from the cell layer as has been described previously78–83. N=3 for all samples.
Supplementary Figure 2 Color changes for standards and samples in the quantification of collagen content.
Hydroxyproline assay (a), Pierce™ BCA Protein assay (b) and Sircol™ Collagen assay (c).
Supplementary Figure 3 Color changes for standards and samples in the quantification of free amines.
TNBSA assay (a) and ninhydrin assay (b).
Supplementary Figure 4 Qualitative/semi-quantitative analysis of enzymatic (MMP-8 at 50 U/ml) degradation of collagen hydrogels and sponges using SDS-PAGE and densitometric analysis.
Non-crosslinked and GTA crosslinked collagen hydrogels and sponges were subjected to MMP-8 degradation for 1 h, 2 h, 4 h and 8 h. Subsequently, the supernatants were collected and loaded on gels [hydrogels (a) and sponges (c)]. Semi-quantitative analysis was conducted through densitometry [hydrogels (b) and sponges (d)]. For the non-crosslinked samples, the degradation is evidenced, whilst the GTA cross-linked samples withheld enzymatic degradation for the period of time assessed.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4. (PDF 698 kb)
Rights and permissions
About this article
Cite this article
Capella-Monsonís, H., Coentro, J., Graceffa, V. et al. An experimental toolbox for characterization of mammalian collagen type I in biological specimens. Nat Protoc 13, 507–529 (2018). https://doi.org/10.1038/nprot.2017.117
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2017.117
This article is cited by
-
Immunogenicity of decellularized extracellular matrix scaffolds: a bottleneck in tissue engineering and regenerative medicine
Biomaterials Research (2023)
-
Collagen constitutes about 12% in females and 17% in males of the total protein in mice
Scientific Reports (2023)
-
In Vitro Collagenase Degradation of Grafts Used Clinically for Anterior Cruciate Ligament Reconstruction: Human Tendon Data
Biomedical Materials & Devices (2023)
-
The influence of animal species, gender and tissue on the structural, biophysical, biochemical and biological properties of collagen sponges
Journal of Materials Science: Materials in Medicine (2021)
-
Enhancement of collagen deposition and cross-linking by coupling lysyl oxidase with bone morphogenetic protein-1 and its application in tissue engineering
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.