Abstract
This protocol is an extension to: Nat. Protoc. 8, 1513–1524 (2013); doi: 10.1038/nprot.2013.090; published online 11 July 2013
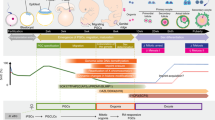
Generation of functional oocytes in culture from pluripotent stem cells should provide a useful model system for improving our understanding of the basic mechanisms underlying oogenesis. In addition, it has potential applications as an alternative source of oocytes for reproduction. Using the most advanced mouse model in regard to reproductive engineering and stem cell biology, we previously developed a culture method that produces functional primorial germ cells starting from pluripotent cells in culture and described it in a previous protocol. This Protocol Extension describes an adaptation of this existing Protocol in which oogenesis also occurs in vitro, thus substantially modifying the technique. Oocytes generated from embryonic stem cells (ESCs) or induced pluripotent stem cells give rise to healthy pups. Here, we describe the protocol for oocyte generation in culture. The protocol is mainly composed of three different culture stages: in vitro differentiation (IVDi), in vitro growth (IVG), and in vitro maturation (IVM), which in total take ∼5 weeks. In each culture period, there are several checkpoints that enable the number of oocytes being produced in the culture to be monitored. The basic structure of the culture system should provide a useful tool for clarifying the complicated sequence of oogenesis in mammals.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hubner, K. et al. Derivation of oocytes from mouse embryonic stem cells. Science 300, 1251–1256 (2003).
Qing, T. et al. Induction of oocyte-like cells from mouse embryonic stem cells by co-culture with ovarian granulosa cells. Differentiation 75, 902–911 (2007).
Salvador, L.M., Silva, C.P., Kostetskii, I., Radice, G.L. & Strauss, J.F. III. The promoter of the oocyte-specific gene, Gdf9, is active in population of cultured mouse embryonic stem cells with an oocyte-like phenotype. Methods 45, 172–181 (2008).
Yu, Z. et al. Dazl promotes germ cell differentiation from embryonic stem cells. J. Mol. Cell Biol. 1, 93–103 (2009).
Hikabe, O. et al. Reconstitution in vitro of the entire cycle of the mouse female germ line. Nature 539, 299–303 (2016).
Zhang, H. & Liu, K. Cellular and molecular regulation of the activation of mammalian primordial follicles: somatic cells initiate follicle activation in adulthood. Hum. Reprod. Update 21, 779–786 (2015).
Edson, M.A., Nagaraja, A.K. & Matzuk, M.M. The mammalian ovary from genesis to revelation. Endocr. Rev. 30, 624–712 (2009).
Hayashi, K. et al. Offspring from oocytes derived from in vitro primordial germ cell-like cells in mice. Science 338, 971–975 (2012).
Hayashi, K., Ohta, H., Kurimoto, K., Aramaki, S. & Saitou, M. Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell 146, 519–532 (2011).
Hayashi, K. & Saitou, M. Generation of eggs from mouse embryonic stem cells and induced pluripotent stem cells. Nat. Protoc. 8, 1513–1524 (2013).
Eppig, J.J. & O'Brien, M.J. Development in vitro of mouse oocytes from primordial follicles. Biol. Reprod. 54, 197–207 (1996).
O'Brien, M.J., Pendola, J.K. & Eppig, J.J. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol. Reprod. 68, 1682–1686 (2003).
Morohaku, K. et al. Complete in vitro generation of fertile oocytes from mouse primordial germ cells. Proc. Natl. Acad. Sci. USA 113, 9021–9026 (2016).
Morohaku, K., Hirao, Y. & Obata, Y. Development of fertile mouse oocytes from mitotic germ cells in vitro. Nat. Protoc. http://dx.doi.org/10.1038/nprot.2017.069 (2017).
Jefferson, W., Newbold, R., Padilla-Banks, E. & Pepling, M. Neonatal genistein treatment alters ovarian differentiation in the mouse: inhibition of oocyte nest breakdown and increased oocyte survival. Biol. Reprod. 74, 161–168 (2006).
Chen, Y., Breen, K. & Pepling, M.E. Estrogen can signal through multiple pathways to regulate oocyte cyst breakdown and primordial follicle assembly in the neonatal mouse ovary. J. Endocrinol. 202, 407–417 (2009).
Dong, J. et al. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature 383, 531–535 (1996).
Yan, C. et al. Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol. Endocrinol. 15, 854–866 (2001).
Hirao, Y. et al. In vitro growth and development of bovine oocyte-granulosa cell complexes on the flat substratum: effects of high polyvinylpyrrolidone concentration in culture medium. Biol. Reprod. 70, 83–91 (2004).
Irie, N. et al. SOX17 is a critical specifier of human primordial germ cell fate. Cell 160, 253–268 (2015).
Sasaki, K. et al. Robust in vitro induction of human germ cell fate from pluripotent stem cells. Cell Stem Cell 17, 178–194 (2015).
Xu, J. et al. Fibrin promotes development and function of macaque primary follicles during encapsulated three-dimensional culture. Hum. Reprod. 28, 2187–2200 (2013).
Tingen, C.M. et al. A macrophage and theca cell-enriched stromal cell population influences growth and survival of immature murine follicles in vitro. Reproduction 141, 809–820 (2011).
Xu, M., Kreeger, P.K., Shea, L.D. & Woodruff, T.K. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 12, 2739–2746 (2006).
Yin, H., Kristensen, S.G., Jiang, H., Rasmussen, A. & Andersen, C.Y. Survival and growth of isolated pre-antral follicles from human ovarian medulla tissue during long-term 3D culture. Hum. Reprod. 31, 1531–1539 (2016).
Matoba, S. & Ogura, A. Generation of functional oocytes and spermatids from fetal primordial germ cells after ectopic transplantation in adult mice. Biol. Reprod. 84, 631–638 (2011).
Nagy, A. Manipulating the Mouse Embryo: A Laboratory Manual 3rd edn. (Cold Spring Harbor Laboratory Press, 2003).
Acknowledgements
We thank N. Hamada, S. Shomamoto, N. Hamazaki and G. Nagamatsu for providing technical details of the protocol. This study was supported in part by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (KAKENHI nos. 25114006, 15K21736, 25290033 and 17H01395); by JST-PRESTO; by the Uehara Memorial Foundation; and by the Takeda Science Foundation.
Author information
Authors and Affiliations
Contributions
K.H., O.H., Y.O. and Y.H. developed the techniques. K.H. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Evaluation of 11 media for IVD culture
Representative images of E12.5 gonads cultured with the media and on the days indicated are shown. Note that the number of oocytes is highest in the culture with SptemPro34. Further evaluation revealed that the combination of αMEM and StemPro34 media was suitable for IVD culture of reconstituted ovaries5. Scale bars, 500 μm. These experiments were performed under the ethical guidelines of Kyushu University.
Supplementary Figure 2 IVD culture with fresh or frozen-thawed gonadal somatic cells
Two representative images of IVDi culture with fresh or frozen-thawed gonadal somatic cells are shown. BF, bright field; SC, stella-ECFP. Scale bars, 200 μm. These experiments were performed under the ethical guidelines of Kyushu University.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1 and 2. (PDF 399 kb)
Rights and permissions
About this article
Cite this article
Hayashi, K., Hikabe, O., Obata, Y. et al. Reconstitution of mouse oogenesis in a dish from pluripotent stem cells. Nat Protoc 12, 1733–1744 (2017). https://doi.org/10.1038/nprot.2017.070
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2017.070
This article is cited by
-
Generation of functional oocytes from male mice in vitro
Nature (2023)
-
In vitro oogenesis from murine premeiotic germ cells using a new three-dimensional culture system
Cell Death Discovery (2023)
-
Eggs made from male mouse stem cells using error-prone culture
Nature (2023)
-
Morphokinetic parameters of mouse oocyte meiotic maturation and cumulus expansion are not affected by reproductive age or ploidy status
Journal of Assisted Reproduction and Genetics (2023)
-
Synergistic effect of Huyang Yangkun Formula and embryonic stem cells on 4-vinylcyclohexene diepoxide induced premature ovarian insufficiency in mice
Chinese Medicine (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.