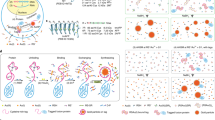
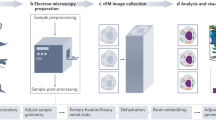
Abstract
Electron microscopy (EM) is the premiere technique for high-resolution imaging of cellular ultrastructure. Unambiguous identification of specific proteins or cellular compartments in electron micrographs, however, remains challenging because of difficulties in delivering electron-dense contrast agents to specific subcellular targets within intact cells. We recently reported enhanced ascorbate peroxidase 2 (APEX2) as a broadly applicable genetic tag that generates EM contrast on a specific protein or subcellular compartment of interest. This protocol provides guidelines for designing and validating APEX2 fusion constructs, along with detailed instructions for cell culture, transfection, fixation, heavy-metal staining, embedding in resin, and EM imaging. Although this protocol focuses on EM in cultured mammalian cells, APEX2 is applicable to many cell types and contexts, including intact tissues and organisms, and is useful for numerous applications beyond EM, including live-cell proteomic mapping. This protocol, which describes procedures for sample preparation from cell monolayers and cell pellets, can be completed in 10 d, including time for APEX2 fusion construct validation, cell growth, and solidification of embedding resins. Notably, the only additional steps required relative to a standard EM sample preparation are cell transfection and a 2- to 45-min staining period with 3,3-diaminobenzidine (DAB) and hydrogen peroxide (H2O2).
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Giepmans, B.N., Adams, S.R., Ellisman, M.H. & Tsien, R.Y. The fluorescent toolbox for assessing protein location and function. Science 312, 217–224 (2006).
Fernandez-Suarez, M. & Ting, A.Y. Fluorescent probes for super-resolution imaging in living cells. Nat. Rev. Mol. Cell Biol. 9, 929–943 (2008).
Xu, K., Shim, S.-H. & Zhuang, X. in Far-Field Optical Nanoscopy (eds. Tinnefeld, P., Eggeling, C. & Hell, S. W.) 27–64 (Springer, 2015).
Eggeling, C. & Hell, S.W. in Far-Field Optical Nanoscopy (eds. Tinnefeld, P., Eggeling, C. & Hell, S. W.) 3–25 (Springer, 2015).
De Mey, J., Moeremans, M., Geuens, G., Nuydens, R. & De Brabander, M. High resolution light and electron microscopic localization of tubulin with the IGS (immuno gold staining) method. Cell Biol. Int. Rep. 5, 889–899 (1981).
Giepmans, B.N.G., Deerinck, T.J., Smarr, B.L., Jones, Y.Z. & Ellisman, M.H. Correlated light and electron microscopic imaging of multiple endogenous proteins using quantum dots. Nat. Methods 2, 743–749 (2005).
Henderson, D. & Weber, K. Three-dimensional organization of microfilaments and microtubules in the cytoskeleton. Immunoperoxidase labelling and stereo-electron microscopy of detergent-extracted cells. Exp. Cell Res. 124, 301–316 (1979).
Deerinck, T.J. et al. Fluorescence photooxidation with eosin: a method for high resolution immunolocalization and in situ hybridization detection for light and electron microscopy. J. Cell Biol. 126, 901–910 (1994).
Schnell, U., Dijk, F., Sjollema, K.A. & Giepmans, B.N.G. Immunolabeling artifacts and the need for live-cell imaging. Nat. Methods 9, 152–158 (2012).
Tokuyasu, K.T. Application of cryoultramicrotomy to immunocytochemistry. J. Microsc. 143, 139–149 (1986).
Kukulski, W. et al. Correlated fluorescence and 3D electron microscopy with high sensitivity and spatial precision. J. Cell Biol. 192, 111 (2011).
Martell, J.D. et al. Engineered ascorbate peroxidase as a genetically encoded reporter for electron microscopy. Nat. Biotechnol. 30, 1143–1148 (2012).
Shu, X. et al. A genetically encoded tag for correlated light and electron microscopy of intact cells, tissues, and organisms. PLoS Biol. 9, e1001041 (2011).
Ariotti, N. et al. Modular detection of GFP-labeled proteins for rapid screening by electron microscopy in cells and organisms. Dev. Cell 35, 513–525 (2015).
Lam, S.S. et al. Directed evolution of APEX2 for electron microscopy and proximity labeling. Nat. Methods 12, 51–54 (2015).
Shvets, E., Bitsikas, V., Howard, G., Hansen, C.G. & Nichols, B.J. Dynamic caveolae exclude bulk membrane proteins and are required for sorting of excess glycosphingolipids. Nat. Commun. 6, 6867 (2015).
Ludwig, A., Nichols, B.J. & Sandin, S. Architecture of the caveolar coat complex. J. Cell Sci. 129, 3077 (2016).
Liu, L.-K., Choudhary, V., Toulmay, A. & Prinz, W.A. An inducible ER–Golgi tether facilitates ceramide transport to alleviate lipotoxicity. J. Cell Biol. 216, 131–147 (2016).
Hyenne, V. et al. RAL-1 controls multivesicular body biogenesis and exosome secretion. J. Cell Biol. 211, 27–37 (2015).
Hung, V. et al. Proteomic mapping of the human mitochondrial intermembrane space in live cells via ratiometric APEX tagging. Mol. Cell 55, 332–341 (2014).
Rhee, H.-W. et al. Proteomic mapping of mitochondria in living cells via spatially restricted enzymatic tagging. Science 339, 1328–1331 (2013).
Bertolin, G. et al. Parkin maintains mitochondrial levels of the protective Parkinson's disease-related enzyme 17-β hydroxysteroid dehydrogenase type 10. Cell Death Differ. 22, 1563–1576 (2015).
Fueller, J. et al. Subcellular partitioning of protein tyrosine phosphatase 1B to the endoplasmic reticulum and mitochondria depends sensitively on the composition of its tail anchor. PLoS One 10, e0139429 (2015).
Lu, Y.-W. et al. Defining functional classes of Barth syndrome mutation in humans. Hum. Mol. Genet. 25, 1754–1770 (2016).
Luo, X. et al. Enhanced transcriptional activity and mitochondrial localization of STAT3 co-induce axon regrowth in the adult central nervous system. Cell Rep. 15, 398–410 (2016).
Long, J.C.D. & Fodor, E. The PB2 subunit of the influenza A virus RNA polymerase is imported into the mitochondrial matrix. J. Virol. 90, 8729–8738 (2016).
Ludwig, A., Nichols, B.J. & Sandin, S. Architecture of the caveolar coat complex. J. Cell Sci. 29, 3077–3083 (2016).
Bohm, U.L. et al. CSF-contacting neurons regulate locomotion by relaying mechanical stimuli to spinal circuits. Nat. Commun. 7, 10866 (2016).
Kotewicz, K.M. et al. A single Legionella effector catalyzes a multistep ubiquitination pathway to rearrange tubular endoplasmic reticulum for replication. Cell Host Microbe 21, 169–181 (2017).
Ellisman, M.H. et al. Advances in molecular probe-based labeling tools and their application to multiscale multimodal correlated microscopies. J. Chem. Biol. 8, 143–151 (2015).
Wong, M. & Munro, S. The specificity of vesicle traffic to the Golgi is encoded in the golgin coiled-coil proteins. Science 346, (2014).
Martell, J.D. et al. A split horseradish peroxidase for the detection of intercellular protein-protein interactions and sensitive visualization of synapses. Nat. Biotechnol. 34, 774–780 (2016).
Salo, V.T. et al. Seipin regulates ER–lipid droplet contacts and cargo delivery. EMBO J. 35, 2699–2716 (2016).
Belevich, I., Joensuu, M., Kumar, D., Vihinen, H. & Jokitalo, E. Microscopy image browser: a platform for segmentation and analysis of multidimensional datasets. PLoS Biol. 14, e1002340 (2016).
Bushong, E.A. et al. X-ray microscopy as an approach to increasing accuracy and efficiency of serial block-face imaging for correlated light and electron microscopy of biological specimens. Microsc. Microanal. 21, 231–238 (2015).
Heusermann, W. et al. Exosomes surf on filopodia to enter cells at endocytic hot spots, traffic within endosomes, and are targeted to the ER. J. Cell Biol. 213, 173–184 (2016).
Su, Y. et al. Mitochondrial transplantation attenuates airway hyperresponsiveness by inhibition of cholinergic hyperactivity. Theranostics 6, 1244–1260 (2016).
Lawrence, A.D. et al. Solution structure of a bacterial microcompartment targeting peptide and its application in the construction of an ethanol bioreactor. ACS Synth. Biol. 3, 454–465 (2014).
Zumthor, J.P. et al. Static clathrin assemblies at the peripheral vacuole—plasma membrane interface of the parasitic protozoan Giardia lamblia. PLoS Pathog. 12, e1005756 (2016).
Lin, T.-Y. et al. Mapping chromatic pathways in the Drosophila visual system. J. Comp. Neurol. 524, 213–227 (2016).
Joesch, M. et al. Reconstruction of genetically identified neurons imaged by serial-section electron microscopy. eLife 5, e15015 (2016).
Eyre, N.S. et al. Phosphorylation of NS5A Serine-235 is essential to hepatitis C virus RNA replication and normal replication compartment formation. Virology 491, 27–44 (2016).
Marceau, C.D. et al. Genetic dissection of Flaviviridae host factors through genome-scale CRISPR screens. Nature 535, 159–163 (2016).
Kittelmann, M., Hawes, C. & Hughes, L. Serial block face scanning electron microscopy and the reconstruction of plant cell membrane systems. J. Microsc. 263, 200–211 (2016).
Jing, J. et al. Proteomic mapping of ER-PM junctions identifies STIMATE as a regulator of Ca2+ influx. Nat. Cell Biol. 17, 1339–1347 (2015).
Chen, C.-L. et al. Proteomic mapping in live Drosophila tissues using an engineered ascorbate peroxidase. Proc. Natl. Acad. Sci. USA 112, 12093–12098 (2015).
Hung, V. et al. Spatially resolved proteomic mapping in living cells with the engineered peroxidase APEX2. Nat. Protoc. 11, 456–475 (2016).
Dwyer, D.J. et al. Antibiotics induce redox-related physiological alterations as part of their lethality. Proc. Natl. Acad. Sci. USA 111, E2100–E2109 (2014).
Choi, H., Yang, Z. & Weisshaar, J.C. Single-cell, real-time detection of oxidative stress induced in Escherichia coli by the antimicrobial peptide CM15. Proc. Natl. Acad. Sci. 112, E303–E310 (2015).
Lee, J. et al. An enhanced ascorbate peroxidase 2/antibody-binding domain fusion protein (APEX2-ABD) as a recombinant target-specific signal amplifier. Chem. Commun. 51, 10945–10948 (2015).
Lee, S.-Y. et al. APEX fingerprinting reveals the subcellular localization of proteins of interest. Cell Rep. 15, 1837–1847 (2016).
Li, J., Wang, Y., Chiu, S.-L. & Cline, H.T. Membrane targeted horseradishperoxidase as a marker for correlative fluorescence and electron microscopy studies. Front. Neural Circuits 4, 6 (2010).
Loh, K.H. et al. Proteomic analysis of unbounded cellular compartments: synaptic clefts. Cell 166, 1295–1307 (2016).
Kuipers, J. et al. FLIPPER, a combinatorial probe for correlated live imaging and electron microscopy, allows identification and quantitative analysis of various cells and organelles. Cell Tissue Res. 360, 61–70 (2015).
Puig, A. & Gilbert, H.F. Protein disulfide isomerase exhibits chaperone and anti-chaperone activity in the oxidative refolding of lysozyme. J. Biol. Chem. 269, 7764–7771 (1994).
Wilson, C.J. & Groves, P.M. Fine structure and synaptic connections of the common spiny neuron of the rat neostriatum: a study employing intracellular injection of horseradish peroxidase. J. Comp. Neurol. 194, 599–615 (1980).
Gaietta, G. et al. Multicolor and electron microscopic imaging of connexin trafficking. Science 296, 503–507 (2002).
Hoffmann, C. et al. Fluorescent labeling of tetracysteine-tagged proteins in intact cells. Nat. Protoc. 5, 1666–1677 (2010).
Jiménez-Banzo, A., Nonell, S., Hofkens, J. & Flors, C. Singlet oxygen photosensitization by EGFP and its chromophore HBDI. Biophys. J. 94, 168–172 (2008).
Mercogliano, C.P. & DeRosier, D.J. Concatenated metallothionein as a clonable gold label for electron microscopy. J. Struct. Biol. 160, 70–82 (2007).
Diestra, E., Fontana, J., Guichard, P., Marco, S. & Risco, C. Visualization of proteins in intact cells with a clonable tag for electron microscopy. J. Struct. Biol. 165, 157–168 (2009).
Sharp, K.H., Mewies, M., Moody, P.C.E. & Raven, E.L. Crystal structure of the ascorbate peroxidase-ascorbate complex. Nat. Struct. Mol. Biol. 10, 303–307 (2003).
Patterson, W.R. & Poulos, T.L. Crystal structure of recombinant pea cytosolic ascorbate peroxidase. Biochemistry 34, 4331–4341 (1995).
Kim, J.H. et al. High cleavage efficiency of a 2A peptide derived from porcine Teschovirus-1 in human cell lines, zebrafish and mice. PLoS One 6, e18556 (2011).
Tiscornia, G., Singer, O. & Verma, I.M. Production and purification of lentiviral vectors. Nat. Protoc. 1, 241–245 (2006).
McIntosh, J.R. Cellular Electron Microscopy 79 (Academic Press, 2011).
Bozzola, J.J. & Russell, L.D. Electron Microscopy: Principles and Techniques for Biologists (Jones & Bartlett Learning, 1999).
Acknowledgements
Funding was provided by the US National Institutes of Health (R01-CA186568 to A.Y.T.; P41 GM103412 and R01GM086197 to M.H.E.) and a Howard Hughes Medical Institute Collaborative Initiative Award (to A.Y.T.). J.D.M. and S.S.L. were supported by National Science Foundation Graduate Research and National Defense Science and Engineering fellowships.
Author information
Authors and Affiliations
Contributions
J.D.M. and A.Y.T. developed the original APEX tag for electron microscopy. S.S.L. and A.Y.T. developed APEX2. T.J.D. and M.H.E. developed protocols for cell staining, EM sample processing, and imaging by light and electron microscopy. J.D.M. prepared all constructs and cell samples for the figures, and T.J.D. performed all EM sample processing and imaging. J.D.M. wrote the paper. All authors edited the paper.
Corresponding authors
Ethics declarations
Competing interests
The Massachusetts Institute of Technology has submitted a patent application related to this work.
Rights and permissions
About this article
Cite this article
Martell, J., Deerinck, T., Lam, S. et al. Electron microscopy using the genetically encoded APEX2 tag in cultured mammalian cells. Nat Protoc 12, 1792–1816 (2017). https://doi.org/10.1038/nprot.2017.065
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2017.065
This article is cited by
-
Deaggregation of mutant Plasmodium yoelii de-ubiquitinase UBP1 alters MDR1 localization to confer multidrug resistance
Nature Communications (2024)
-
Using the heme peroxidase APEX2 to probe intracellular H2O2 flux and diffusion
Nature Communications (2024)
-
Spatial and functional arrangement of Ebola virus polymerase inside phase-separated viral factories
Nature Communications (2023)
-
Subcellular distribution of the rAAV genome depends on genome structure
Scientific Reports (2023)
-
Ultrastruktur in 3D: neue Ansichten und Einsichten in der Zellbiologie
BIOspektrum (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.