Abstract
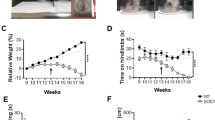
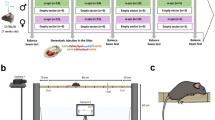
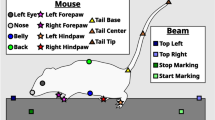
Phenotypic analysis of mouse models of human diseases is essential to understanding the underlying disease mechanisms and to developing therapeutics. Many models of neurodegenerative diseases are associated with motor dysfunction, a powerful readout for the disease. We describe here a set of measures to quantitatively monitor early disease onset and progression. We named this set of rules qMotor because it enables sensitive, robust and quantitative measurement of motor performance in 3 d. qMotor can be used to assess early disease onset, before paralysis, as well as disease progression in diverse mouse models, and can be exploited to define robust and humane experimental end points, thereby reducing animal suffering. As an example, we apply qMotor to SOD1G93A transgenic mice. Early studies with the original transgenic SOD1G93A mice in the hybrid background (B6SJL-Tg(SOD1-G93A) have been criticized because of high noise in this mixed background and because of inadequate study designs. We applied qMotor in SOD1G93A transgenic mice in an inbred C57BL/6J background, hereafter called iSOD1G93A mice, and show a remarkably robust and consistent phenotype in this line that we use to evaluate a therapeutic approach. qMotor is a protocol generically applicable to different mouse models.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Jucker, M. The benefits and limitations of animal models for translational research in neurodegenerative diseases. Nat. Med. 16, 1210–1214 (2010).
Mead, R.J. et al. Optimised and rapid pre-clinical screening in the SOD1(G93A) transgenic mouse model of amyotrophic lateral sclerosis (ALS). PLoS One 6, e23244 (2011).
Das, I. et al. Preventing proteostasis diseases by selective inhibition of a phosphatase regulatory subunit. Science 348, 239–242 (2015).
Ludolph, A.C. et al. Guidelines for preclinical animal research in ALS/MND: a consensus meeting. Amyotroph. Lateral Scler. 11, 38–45 (2010).
Buitrago, M. Short and long-term motor skill learning in an accelerated rotarod training paradigm. Neurobiol. Learn. Mem. 81, 211–216 (2004).
Brooks, S.P. & Dunnett, S.B. Tests to assess motor phenotype in mice: a user's guide. Nat. Rev. Neurosci. 10, 519–529 (2009).
Wrabetz, L. et al. Different intracellular pathomechanisms produce diverse Myelin Protein Zero neuropathies in transgenic mice. J. Neurosci. 26, 2358–2368 (2006).
Pennuto, M. et al. Ablation of the UPR-mediator CHOP restores motor function and reduces demyelination in Charcot-Marie-Tooth 1B mice. Neuron 57, 393–405 (2008).
D'Antonio, M. et al. Resetting translational homeostasis restores myelination in Charcot-Marie-Tooth disease type 1B mice. J. Exp. Med. 210, 821–838 (2013).
Scott, S. et al. Design, power, and interpretation of studies in the standard murine model of ALS. Amyotroph. Lateral Scler. 9, 4–15 (2008).
Gurney, M.E. et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science 264, 1772–1775 (1994).
Hall, E.D., Oostveen, J.A. & Gurney, M.E. Relationship of microglial and astrocytic activation to disease onset and progression in a transgenic model of familial ALS. Glia 23, 249–256 (1998).
Benatar, M. Lost in translation:treatment trials in the SOD1 mouse and in human ALS. Neurobiol. Dis. 26, 1–13 (2007).
Ralph, G.S. et al. Silencing mutant SOD1 using RNAi protects against neurodegeneration and extends survival in an ALS model. Nat. Med. 11, 429–433 (2005).
Kilkenny, C., Browne, W.J., Cuthill, I.C., Emerson, M. & Altman, D.G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 8, e1000412 (2010).
Acknowledgements
We thank A. Devoy, for the generous gift of the iSOD1G93A C57BL/6J line and for her support in establishing the colony in our facility; L. Wrabetz and M. D'Antonio of the San Raffaele Institute, for the CMT-1B mice; the LMB mouse facility; A. Segonds-Pichon, for advice on statistical analyses; members of the Bertolotti lab for discussions; and J. Watson, for comments on the manuscript. A.B. is an honorary fellow of the Clinical Neurosciences Department of Cambridge University. This work was supported by the Medical Research Council and the European Research Council under the European Union's Seventh Framework Programme (FP7/2007-2013)/ERC grant 309516.
Author information
Authors and Affiliations
Contributions
L.M.L. wrote and validated the protocol. I.D. performed the previously published experiments3. L.M.L. and A.B. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Rotarod analysis of CMT-1B mice separated by gender
a,b, Rotarod analysis of wild-type or CMT-1B females (a) or males (b) mice of indicated age following orally gavage (twice daily) with Sephin1 (1 mg/kg) or vehicle. Data are means of three trails performed on the same day ± SEM (n=4-8). The data shown here are the same as in Figure 1 but the analysis were performed separating genders. No samples, mice or data points were excluded from the analyses. All data shown were collected while conforming to governmental and institutional guidelines for care and use of laboratory animals. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001; (males F(1,14)=13.55, p=0.0025; females F(1,14)=9.38, p=0.0091).
Supplementary Figure 2 Rotarod analysis of iSODG93A mice separated by genders
a,b, Rotarod analysis of wild-type or iSOD1G93A mice of indicated age and gender treated orally with Sephin1 (5 mg/kg) or vehicle once a day from 28 days of age. Data are means of 3 trails performed on the same day ± SEM (n=2-3). The data shown here are the same as in Figure 2 but the analysis were performed separating genders. No samples, mice or data points were excluded from the analyses. All data shown were collected while conforming to governmental and institutional guidelines for care and use of laboratory animals.
Supplementary Figure 3 Rotarod analysis of iSODG93A mice at 90 days of age.
a-c, Rotarod analysis of wild-type or iSOD1G93A mice of indicated age (days). (a): mixed genders, (b): females and (c) males. Data are means of 3 trials performed on three consecutive days ± SEM (n=11-15). No samples, mice or data points were excluded from the analyses. All data shown were collected while conforming to governmental and institutional guidelines for care and use of laboratory animals. All data shown were collected while conforming to governmental and institutional guidelines for care and use of laboratory animals. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001; (a. F(1,24)=199.0, p<0.0001; b. F(1,12)=104.3, p<0.0001, c. F(1,10)=114.1, p<0.0001).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3. (PDF 452 kb)
Supplementary Table 1
nprot.2017.062_SuppTable1_revJC. (XLSX 11 kb)
Supplementary Table 2
nprot.2017.062_SuppTable2_revJC. (XLSX 13 kb)
Supplementary Table 3
nprot.2017.062_SuppTable3_revJC. (XLSX 10 kb)
Rights and permissions
About this article
Cite this article
Luh, L., Das, I. & Bertolotti, A. qMotor, a set of rules for sensitive, robust and quantitative measurement of motor performance in mice. Nat Protoc 12, 1451–1457 (2017). https://doi.org/10.1038/nprot.2017.062
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2017.062
This article is cited by
-
Inhibition of carnitine palmitoyl-transferase 1 is a potential target in a mouse model of Parkinson’s disease
npj Parkinson's Disease (2023)
-
Modulation of histone H3K4 dimethylation by spermidine ameliorates motor neuron survival and neuropathology in a mouse model of ALS
Journal of Biomedical Science (2022)
-
Targeting thalamic circuits rescues motor and mood deficits in PD mice
Nature (2022)
-
Downregulating carnitine palmitoyl transferase 1 affects disease progression in the SOD1 G93A mouse model of ALS
Communications Biology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.