Abstract
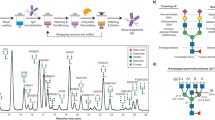
Glycoengineered therapeutic antibodies and glycosite-specific antibody–drug conjugates (gsADCs) have generated great interest among researchers because of their therapeutic potential. Endoglycosidase-catalyzed in vitro glycoengineering technology is a powerful tool for IgG Fc (fragment cystallizable) N-glycosylation remodeling. In this protocol, native heterogeneously glycosylated IgG N-glycans are first deglycosylated with a wild-type endoglycosidase. Next, a homogeneous N-glycan substrate, presynthesized as described here, is attached to the remaining N-acetylglucosamine (GlcNAc) of IgG, using a mutant endoglycosidase (also called endoglycosynthase) that lacks hydrolytic activity but possesses transglycosylation activity for glycoengineering. Compared with in vivo glycoengineering technologies and the glycosyltransferase-enabled in vitro engineering method, the current approach is robust and features quantitative yield, homogeneous glycoforms of produced antibodies and ADCs, compatibility with diverse natural and non-natural glycan structures, convenient exploitation of native IgG as the starting material, and a well-defined conjugation site for antibody modifications. Potential applications of this method cover a broad scope of antibody-related research, including the development of novel glycoengineered therapeutic antibodies with enhanced efficacy, site-specific antibody–drug conjugation, and site-specific modification of antibodies for fluorescent labeling, PEGylation, protein cross-linking, immunoliposome formation, and so on, without loss of antigen-binding affinity. It takes 5–8 d to prepare the natural or modified N-glycan substrates, 3–4 d to engineer the IgG N-glycosylation, and 2–5 d to synthesize the small-molecule toxins and prepare the gsADCs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Shields, R.L. et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J. Biol. Chem. 277, 26733–26740 (2002).
Yamane-Ohnuki, N. et al. Establishment of FUT8 knockout Chinese hamster ovary cells: an ideal host cell line for producing completely defucosylated antibodies with enhanced antibody-dependent cellular cytotoxicity. Biotechnol. Bioeng. 87, 614–622 (2004).
Shinkawa, T. et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J. Biol. Chem. 278, 3466–3473 (2003).
Gramer, M.J. et al. Modulation of antibody galactosylation through feeding of uridine, manganese chloride, and galactose. Biotechnol. Bioeng. 108, 1591–1602 (2011).
Anthony, R.M. et al. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science 320, 373–376 (2008).
Anthony, R.M., Wermeling, F., Karlsson, M.C. & Ravetch, J.V. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc. Natl. Acad. Sci. USA 105, 19571–19578 (2008).
Kaneko, Y., Nimmerjahn, F. & Ravetch, J.V. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science 313, 670–673 (2006).
Liu, L. Antibody glycosylation and its impact on the pharmacokinetics and pharmacodynamics of monoclonal antibodies and Fc-fusion proteins. J. Pharm. Sci. 104, 1866–1884 (2015).
Goetze, A.M. et al. High-mannose glycans on the Fc region of therapeutic IgG antibodies increase serum clearance in humans. Glycobiology 21, 949–959 (2011).
Beck, A. & Reichert, J.M. Marketing approval of mogamulizumab: a triumph for glyco-engineering. MAbs 4, 419–425 (2012).
Sondermann, P. & Szymkowski, D.E. Harnessing Fc receptor biology in the design of therapeutic antibodies. Curr. Opin. Immunol. 40, 78–87 (2016).
DiLillo, D.J. & Ravetch, J.V. Fc-Receptor interactions regulate both cytotoxic and immunomodulatory therapeutic antibody effector functions. Cancer Immunol. Res. 3, 704–713 (2015).
Brandsma, A.M., Jacobino, S.R., Meyer, S., ten Broeke, T. & Leusen, J.H. Fc receptor inside-out signaling and possible impact on antibody therapy. Immunol. Rev. 268, 74–87 (2015).
Ferrara, C. et al. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose. Proc. Natl. Acad. Sci. USA 108, 12669–12674 (2011).
Yamane-Ohnuki, N. & Satoh, M. Production of therapeutic antibodies with controlled fucosylation. MAbs 1, 230–236 (2009).
Umana, P., Jean-Mairet, J., Moudry, R., Amstutz, H. & Bailey, J.E. Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody-dependent cellular cytotoxic activity. Nat. Biotechnol. 17, 176–180 (1999).
Warnock, D. et al. In vitro galactosylation of human IgG at 1 kg scale using recombinant galactosyltransferase. Biotechnol. Bioeng. 92, 831–842 (2005).
Raju, T.S., Briggs, J.B., Chamow, S.M., Winkler, M.E. & Jones, A.J. Glycoengineering of therapeutic glycoproteins: in vitro galactosylation and sialylation of glycoproteins with terminal N-acetylglucosamine and galactose residues. Biochemistry 40, 8868–8876 (2001).
Li, B., Song, H., Hauser, S. & Wang, L.X. A highly efficient chemoenzymatic approach toward glycoprotein synthesis. Org. Lett. 8, 3081–3084 (2006).
Huang, W. et al. Glycosynthases enable a highly efficient chemoenzymatic synthesis of N-glycoproteins carrying intact natural N-glycans. J. Am. Chem. Soc. 131, 2214–2223 (2009).
Goodfellow, J.J. et al. An endoglycosidase with alternative glycan specificity allows broadened glycoprotein remodelling. J. Am. Chem. Soc. 134, 8030–8033 (2012).
Huang, W., Giddens, J., Fan, S.Q., Toonstra, C. & Wang, L.X. Chemoenzymatic glycoengineering of intact IgG antibodies for gain of functions. J. Am. Chem. Soc. 134, 12308–12318 (2012).
Lin, C.W. et al. A common glycan structure on immunoglobulin G for enhancement of effector functions. Proc. Natl. Acad. Sci. USA 112, 10611–10616 (2015).
Parsons, T.B. et al. Optimal synthetic glycosylation of a therapeutic antibody. Angew. Chem. Int. Ed. Engl. 55, 2361–2367 (2016).
Li, T.Z., Tong, X., Yang, Q., Giddens, J.P. & Wang, L.X. Glycosynthase mutants of endoglycosidase S2 show potent transglycosylation activity and remarkably relaxed substrate specificity for antibody glycosylation remodeling. J. Biol. Chem. 291, 16508–16518 (2016).
Feng, T. et al. One-pot N-glycosylation remodeling of IgG with non-natural sialylglycopeptides enables glycosite-specific and dual-payload antibody-drug conjugates. Org. Biomol. Chem. 14, 9501–9518 (2016).
Priyanka, P., Parsons, T.B., Miller, A., Platt, F.M. & Fairbanks, A.J. Chemoenzymatic synthesis of a phosphorylated glycoprotein. Angew. Chem. Int. Ed. Engl. 55, 5058–5061 (2016).
Umekawa, M. et al. Mutants of Mucor hiemalis endo-beta-N-acetylglucosaminidase show enhanced transglycosylation and glycosynthase-like activities. J. Biol. Chem. 283, 4469–4479 (2008).
Sjogren, J. et al. EndoS and EndoS2 hydrolyze Fc-glycans on therapeutic antibodies with different glycoform selectivity and can be used for rapid quantification of high-mannose glycans. Glycobiology 25, 1053–1063 (2015).
Li, X., Fang, T. & Boons, G.J. Preparation of well-defined antibody-drug conjugates through glycan remodeling and strain-promoted azide-alkyne cycloadditions. Angew. Chem. Int. Ed. Engl. 53, 7179–7182 (2014).
Zhou, Q. et al. Site-specific antibody-drug conjugation through glycoengineering. Bioconjug. Chem. 25, 510–520 (2014).
Zhu, Z. et al. Site-specific antibody-drug conjugation through an engineered glycotransferase and a chemically reactive sugar. MAbs 6, 1190–1200 (2014).
Tumbale, P., Jamaluddin, H., Thiyagarajan, N., Acharya, K.R. & Brew, K. Screening a limited structure-based library identifies UDP-GalNAc-specific mutants of alpha-1,3-galactosyltransferase. Glycobiology 18, 1036–1043 (2008).
Li, H. et al. Optimization of humanized IgGs in glycoengineered Pichia pastoris. Nat. Biotechnol. 24, 210–215 (2006).
Junutula, J.R. et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat. Biotechnol. 26, 925–932 (2008).
Zimmerman, E.S. et al. Production of site-specific antibody-drug conjugates using optimized non-natural amino acids in a cell-free expression system. Bioconjug. Chem. 25, 351–361 (2014).
Sun, B. et al. A simplified procedure for gram-scale production of sialylglycopeptide (SGP) from egg yolks and subsequent semi-synthesis of Man3GlcNAc oxazoline. Carbohydr. Res. 396, 62–69 (2014).
Maki, Y., Okamoto, R., Izumi, M., Murase, T. & Kajihara, Y. Semisynthesis of intact complex-type triantennary oligosaccharides from a biantennary oligosaccharide isolated from a natural source by selective chemical and enzymatic glycosylation. J. Am. Chem. Soc. 138, 3461–3468 (2016).
Yamamoto, K., Kadowaki, S., Fujisaki, M., Kumagai, H. & Tochikura, T. Novel specificities of Mucor hiemalis endo-beta-N-acetylglucosaminidase acting complex asparagine-linked oligosaccharides. Biosci. Biotechnol. Biochem. 58, 72–77 (1994).
Noguchi, M., Tanaka, T., Gyakushi, H., Kobayashi, A. & Shoda, S. Efficient synthesis of sugar oxazolines from unprotected N-acetyl-2-amino sugars by using chloroformamidinium reagent in water. J. Org. Chem. 74, 2210–2212 (2009).
Ning, X.H., Guo, J., Wolfert, M.A. & Boons, G.J. Visualizing metabolically labeled glycoconjugates of living cells by copper-free and fast huisgen cycloadditions. Angew. Chem. Int. Ed. Engl. 47, 2253–2255 (2008).
Baskin, J.M. et al. Copper-free click chemistry for dynamic in vivo imaging. Proc. Natl. Acad. Sci. USA 104, 16793–16797 (2007).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NNSFC, nos. 21372238 and 21572244 to W.H.), the SIMM Institute Fund (CASIMM0120153004 to W.H.), and the 'Personalized Medicines: Molecular Signature-based Drug Discovery and Development' Strategic Priority Research Program of the Chinese Academy of Sciences (grant no. XDA12020311 to W.H.).
Author information
Authors and Affiliations
Contributions
F.T. and W.H. designed the research and developed the methods; F.T. performed the experiments; F.T. and W.H. wrote the manuscript; and L.-X.W. developed the original IgG glycoengineering approach and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Methods and Supplementary Results. (PDF 7521 kb)
Rights and permissions
About this article
Cite this article
Tang, F., Wang, LX. & Huang, W. Chemoenzymatic synthesis of glycoengineered IgG antibodies and glycosite-specific antibody–drug conjugates. Nat Protoc 12, 1702–1721 (2017). https://doi.org/10.1038/nprot.2017.058
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2017.058
This article is cited by
-
Selective N-glycan editing on living cell surfaces to probe glycoconjugate function
Nature Chemical Biology (2020)
-
MIgGGly (mouse IgG glycosylation analysis) - a high-throughput method for studying Fc-linked IgG N-glycosylation in mice with nanoUPLC-ESI-MS
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.