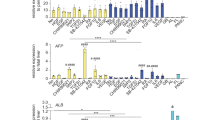
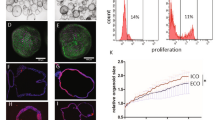
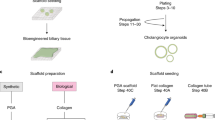
Abstract
The difficulty in isolating and propagating functional primary cholangiocytes is a major limitation in the study of biliary disorders and the testing of novel therapeutic agents. To overcome this problem, we have developed a platform for the differentiation of human pluripotent stem cells (hPSCs) into functional cholangiocyte-like cells (CLCs). We have previously reported that our 26-d protocol closely recapitulates key stages of biliary development, starting with the differentiation of hPSCs into endoderm and subsequently into foregut progenitor (FP) cells, followed by the generation of hepatoblasts (HBs), cholangiocyte progenitors (CPs) expressing early biliary markers and mature CLCs displaying cholangiocyte functionality. Compared with alternative protocols for biliary differentiation of hPSCs, our system does not require coculture with other cell types and relies on chemically defined conditions up to and including the generation of CPs. A complex extracellular matrix is used for the maturation of CLCs; therefore, experience in hPSC culture and 3D organoid systems may be necessary for optimal results. Finally, the capacity of our platform for generating large amounts of disease-specific functional cholangiocytes will have broad applications for cholangiopathies, in disease modeling and for screening of therapeutic compounds.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
O'Hara, S.P., Tabibian, J.H., Splinter, P.L. & Larusso, N.F. The dynamic biliary epithelia: molecules, pathways, and disease. J. Hepatol. 58, 575–582 (2013).
Park, S.M. The crucial role of cholangiocytes in cholangiopathies. Gut Liver 6, 295–304 (2012).
Lazaridis, K.N. The cholangiopathies Konstantinos. Mayo Clin. Proc. 90, 791–800 (2015).
Pollheimer, M.J., Trauner, M. & Fickert, P. Will we ever model PSC? - 'It's hard to be a PSC model!'. Clin. Res. Hepatol. Gastroenterol. 35, 792–804 (2011).
Lemaigre, F.P. Notch signaling in bile duct development: new insights raise new questions. Hepatology 48, 358–360 (2008).
Sampaziotis, F. et al. Cholangiocytes derived from human induced pluripotent stem cells for disease modeling and drug validation. Nat. Biotechnol. 33, 845–852 (2015).
Si-Tayeb, K., Lemaigre, F.P. & Duncan, S.A. Organogenesis and development of the liver. Dev. Cell 18, 175–189 (2010).
Hannan, N.R.F., Segeritz, C.-P., Touboul, T. & Vallier, L. Production of hepatocyte-like cells from human pluripotent stem cells. Nat. Protoc. 8, 430–437 (2013).
Clotman, F. et al. Control of liver cell fate decision by a gradient of TGF beta signaling modulated by Onecut transcription factors. Genes Dev. 19, 1849–1854 (2005).
Yanai, M. et al. FGF signaling segregates biliary cell-lineage from chick hepatoblasts cooperatively with BMP4 and ECM components in vitro. Dev. Dyn. 237, 1268–1283 (2008).
Tabibian, J.H., Masyuk, A.I., Masyuk, T.V., O'Hara, S.P. & LaRusso, N.F. Physiology of cholangiocytes. Compr. Physiol. 3, 541–565 (2013).
Ogawa, M. et al. Directed differentiation of cholangiocytes from human pluripotent stem cells. Nat. Biotechnol. 33, 853–861 (2015).
Tanimizu, N., Miyajima, A. & Mostov, K.E. Liver progenitor cells develop cholangiocyte-type epithelial polarity in three-dimensional culture. Mol. Biol. Cell 18, 1472–1479 (2007).
Zhao, D. et al. Derivation and characterization of hepatic progenitor cells from human embryonic stem cells. PLoS One 4, e6468 (2009).
Tabibian, J.H. et al. Characterization of cultured cholangiocytes isolated from livers of patients with primary sclerosing cholangitis. Lab. Invest. 94, 1126–1133 (2014).
Grant, A.G. & Billing, B.H. The isolation and characterization of a bile ductule cell population from normal and bile-duct ligated rat livers. Br. J. Exp. Pathol. 58, 301–310 (1977).
Joplin, R., Strain, A.J. & Neuberger, J.M. Immuno-isolation and culture of biliary epithelial cells from normal human liver. In Vitro Cell. Dev. Biol. 25, 1189–1192 (1989).
Zaret, K.S. et al. Directed differentiation of cholangiocytes from human pluripotent stem cells. Eur. J. Cell Biol. 33, 1–48 (2015).
Dianat, N. et al. Generation of functional cholangiocyte-like cells from human pluripotent stem cells and HepaRG cells. Hepatology 60, 700–714 (2014).
Glaser, S. et al. Heterogeneity of the intrahepatic biliary epithelium. World J. Gastroenterol. 12, 3523–3536 (2006).
Kent, L. Culture and maintenance of human embryonic stem cells. J. Vis. Exp. 2–5 (2009).
Agudo, J. et al. GFP-specific CD8 T cells enable targeted cell depletion and visualization of T-cell interactions. Nat. Biotechnol. 33, 1287–1292 (2015).
Acknowledgements
This work was funded by ERC starting grant Relieve IMDs (L.V., N.R.F.H.), the Cambridge Hospitals National Institute for Health Research Biomedical Research Center (L.V., N.R.F.H., F.S.), the Evelyn trust (N.R.F.H.) and the EU Fp7 grant TissuGEN (M.C.d.B.). F.S. was supported by an Addenbrooke's Charitable Trust Clinical Research Training Fellowship and a joint MRC-Sparks Clinical Research Training Fellowship.
The authors thank the Cambridge BRC hIPSCs core facility for the derivation of the Cystic Fibrosis hIPSC line, P. Materek (Wellcome Trust-Medical Research Council Stem Cell Institute, Cambridge Stem Cell Institute, Anne McLaren Laboratory, Department of Surgery, University of Cambridge) for the provision of cells used as negative controls, D. Ortmann for his input into the design of the figures and P.-A. Tsagkaraki for her help with the generation of the manuscript figures and statistical analyses.
Author information
Authors and Affiliations
Contributions
F.S.: design and concept of study, execution of experiments and data acquisition, development of protocols and validation, collection of data, production of figures, manuscript writing and editing, and final approval of the manuscript. M.C.d.B., I.G. and A.B.: execution of experiments, collection and provision of data. N.R.F.H.: design and concept of study, editing and final approval of the manuscript. L.V.: design and concept of the study, editing and final approval of the manuscript. M.C.d.B., I.G. and A.B. contributed equally to this work. L.V. and N.R.F.H. jointly directed this work, contributing equally.
Corresponding author
Ethics declarations
Competing interests
L.V. is a founder and shareholder of DefiniGEN. The remaining authors have nothing to disclose.
Integrated supplementary information
Supplementary Figure 1 Morphology of CLC organoids
CLC organoids exhibit a typical cystic or branching tubular morphology. The black arrow indicates a tubular organoid, while the white arrow indicates a branching point. Scale bars: 100μm
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1 and 2 (PDF 412 kb)
Rights and permissions
About this article
Cite this article
Sampaziotis, F., de Brito, M., Geti, I. et al. Directed differentiation of human induced pluripotent stem cells into functional cholangiocyte-like cells. Nat Protoc 12, 814–827 (2017). https://doi.org/10.1038/nprot.2017.011
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2017.011
This article is cited by
-
Three Dimensional Bioprinting for Hepatic Tissue Engineering: From In Vitro Models to Clinical Applications
Tissue Engineering and Regenerative Medicine (2024)
-
Modelling metabolic diseases and drug response using stem cells and organoids
Nature Reviews Endocrinology (2022)
-
Human biomimetic liver microphysiology systems in drug development and precision medicine
Nature Reviews Gastroenterology & Hepatology (2021)
-
Self-organization of organoids from endoderm-derived cells
Journal of Molecular Medicine (2021)
-
Synthetic alternatives to Matrigel
Nature Reviews Materials (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.