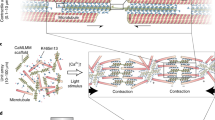
Abstract
Biological machines consisting of cells and biomaterials have the potential to dynamically sense, process, respond, and adapt to environmental signals in real time. As a first step toward the realization of such machines, which will require biological actuators that can generate force and perform mechanical work, we have developed a method of manufacturing modular skeletal muscle actuators that can generate up to 1.7 mN (3.2 kPa) of passive tension force and 300 μN (0.56 kPa) of active tension force in response to external stimulation. Such millimeter-scale biological actuators can be coupled to a wide variety of 3D-printed skeletons to power complex output behaviors such as controllable locomotion. This article provides a comprehensive protocol for forward engineering of biological actuators and 3D-printed skeletons for any design application. 3D printing of the injection molds and skeletons requires 3 h, seeding the muscle actuators takes 2 h, and differentiating the muscle takes 7 d.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Melchels, F.P.W., Feijen, J. & Grijpma, D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials 31, 6121–6130 (2010).
Raman, R. & Bashir, R. Stereolithographic 3D bioprinting for biomedical applications. in Essentials of 3D Biofabrication and Translation (eds. Atala, A. & Yoo, J.J.) Ch. 6 (Academic Press) 89–121 (2015).
Raman, R. et al. High-resolution projection microstereolithography for patterning of neovasculature. Adv. Healthc. Mater. 5, 610–619 (2016).
Sears, N.A. A review of 3D printing in tissue engineering. Tissue Eng. Part B Rev. 22, 298–310 (2016).
Peltola, S.M., Grijpma, D.W., Melchels, F.P.W. & Kellomaki, M. A review of rapid prototyping techniques for tissue engineering purposes. Ann. Med. 40, 268–280 (2008).
Bajaj, P., Schweller, R.M., Khademhosseini, A., West, J.L. & Bashir, R. 3D biofabrication strategies for tissue engineering and regenerative medicine. Annu. Rev. Biomed. Eng. 16, 247–276 (2013).
Kamm, R.D. & Bashir, R. Creating living cellular machines. Ann. Biomed. Eng. 42, 445–459 (2014).
Feinberg, A.W. Biological soft robotics. Annu. Rev. Biomed. Eng. 17, 243–65 (2015).
Chan, V., Asada, H.H. & Bashir, R. Utilization and control of bioactuators across multiple length scales. Lab Chip 14, 653–670 (2014).
Sambasivan, R. & Tajbakhsh, S. Vertebrate Myogenesis 56, 191–213 (2015).
Duffy, R.M. & Feinberg, A.W. Engineered skeletal muscle tissue for soft robotics: fabrication strategies, current applications, and future challenges. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 6, 178–195 (2014).
Bian, W., Liau, B., Badie, N. & Bursac, N. Mesoscopic hydrogel molding to control the 3D geometry of bioartificial muscle tissues. Nat. Protoc. 4, 1522–1534 (2009).
Cvetkovic, C. et al. Three-dimensionally printed biological machines powered by skeletal muscle. Proc. Natl. Acad. Sci. USA 111, 10125–10130 (2014).
Raman, R. et al. Optogenetic skeletal muscle-powered adaptive biological machines. Proc. Natl. Acad. Sci. USA 113, 3497–3502 (2016).
Feinberg, A.W. et al. Muscular thin films for building actuators and powering devices. Science 317, 1366–70 (2007).
Nawroth, J.C. et al. A tissue-engineered jellyfish with biomimetic propulsion. Nat. Biotechnol. 30, 792–797 (2012).
Chan, V. et al. Multi-material bio-fabrication of hydrogel cantilevers and actuators with stereolithography. Lab Chip 12, 88–98 (2012).
Chan, V. et al. Development of miniaturized walking biological machines. Sci. Rep. 2, 857 (2012).
Park, S.-J. et al. Phototactic guidance of a tissue-engineered soft-robotic ray. Science 353, 158–162 (2016).
Bian, W. & Bursac, N. Engineered skeletal muscle tissue networks with controllable architecture. Biomaterials 30, 1401–1412 (2009).
Hinds, S., Bian, W., Dennis, R.G. & Bursac, N. The role of extracellular matrix composition in structure and function of bioengineered skeletal muscle. Biomaterials 32, 3575–83 (2011).
Sakar, M.S. et al. Formation and optogenetic control of engineered 3D skeletal muscle bioactuators. Lab Chip 12, 4976–85 (2012).
Rangarajan, S., Madden, L. & Bursac, N. Use of flow, electrical, and mechanical stimulation to promote engineering of striated muscles. Ann. Biomed. Eng. 42, 1391–1405 (2014).
Dennis, R.G., Kosnik, P.E., Gilbert, M.E. & Faulkner, J.A. Excitability and contractility of skeletal muscle engineered from primary cultures and cell lines. Am. J. Physiol. Cell Physiol. 280, C288–C295 (2001).
Herr, H. & Dennis, R.G. A swimming robot actuated by living muscle tissue. J. Neuroeng. Rehabil. 1, 6 (2004).
Kaur, G. & Dufour, J.M. Cell lines: valuable tools or useless artifacts. Spermatogenesis 2, 1–5 (2012).
Chan, V., Zorlutuna, P., Jeong, J.H., Kong, H. & Bashir, R. Three-dimensional photopatterning of hydrogels using stereolithography for long-term cell encapsulation. Lab Chip 10, 2062–2070 (2010).
Neiman, J.A.S. et al. Photopatterning of hydrogel scaffolds coupled to filter materials using stereolithography for perfused 3D culture of hepatocytes. Biotechnol. Bioeng. 112, 777–787 (2015).
Raman, R. et al. 3D printing enables separation of orthogonal functions within a hydrogel particle. Biomed. Microdevices 18, 49 (2016).
Novosel, E.C., Kleinhans, C. & Kluger, P.J. Vascularization is the key challenge in tissue engineering. Adv. Drug Deliv. Rev. 63, 300–311 (2011).
Barolet, D. Light-emitting diodes (LEDs) in dermatology. Semin. Cutan. Med. Surg. 27, 227–238 (2008).
Moreira, M.C., Prado, R. & Campos, A. Application of high brightness LEDs in the human tissue and its therapeutic response. in Applied Biomedical Engineering (eds. Gargiulo, G.D. & McEwan, A.) Ch. 1 (InTech) 3–20 (2011).
Donnelly, K. et al. A novel bioreactor for stimulating skeletal muscle in vitro. Tissue Eng. Part C Methods 16, 711–718 (2010).
Powell, C.A., Smiley, B.L., Mills, J. & Vandenburgh, H.H. Mechanical stimulation improves tissue-engineered human skeletal muscle. Am. J. Physiol. Cell Physiol. 283, C1557–C1565 (2002).
Duan, C., Ren, H. & Gao, S. Insulin-like growth factors (IGFs), IGF receptors, and IGF-binding proteins: roles in skeletal muscle growth and differentiation. Gen. Comp. Endocrinol. 167, 344–351 (2010).
Uzel, S.G.M. et al. Microfluidic platform for the formation of optically excitable, three-dimensional, compartmentalized motor units. Sci. Adv. 2, e1501429 (2015).
Cordeli, F. Manual Tracking. https://imagej.nih.gov/ij/plugins/track/track.html (2005).
Acknowledgements
We thank J. Sinn-Hanlon at the University of Illinois at Urbana-Champaign (UIUC) for image rendering of Figure 1a, and the Core Facilities at the Carl R. Woese Institute for Genomic Biology at the University of Illinois at Urbana-Champaign for assistance with sample preparation and imaging. We also thank V. Chan, P. Bajaj, S. Uzel, P. Sengupta, T. Saif, and R. Kamm for insightful discussions regarding this work. This work was funded by National Science Foundation (NSF) Science and Technology Center Emergent Behavior of Integrated Cellular Systems (EBICS) Grant CBET – 0939511. R.R. was funded by NSF Graduate Research Fellowship Grant DGE – 1144245. R.R. and C.C. were funded by an NSF Cellular and Molecular Mechanics and Bionanotechnology (CMMB) Integrative Graduate Education and Research Traineeship (IGERT) at UIUC (grant 0965918).
Author information
Authors and Affiliations
Contributions
R.R. designed the modular muscle-ring protocol; R.R. performed and analyzed muscle seeding and functional assessment experiments; C.C. performed and analyzed muscle staining experiments and protein quantification experiments; R.R., C.C., and R.B. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Cellular alignment and circularity analysis
Starting with a fluorescent image of cellular nuclei, convert the image to binary. To calculate circularity, apply a threshold to the binary image and use the Analyze Particles feature in ImageJ to bin and plot the circularity (1 = circular; 0 = linear) for each data set. To compute alignment, perform FFT analysis on similarly-oriented images and plot the results (radial sums) as a function of degrees. (See Supplementary Methods for more information.) Circularity plots represent all data points from Figure 2c (n=2312 total nuclei for muscle rings; n=2702 for muscle strips). Data from normal distributions represent mean values ± standard deviations; * = p < 0.05. FFT Alignment plots represent individual curves for muscle ring and strip samples; averaged data are shown in bold black lines on each plot (and plotted together for comparison in Figure 2b).
Supplementary Figure 2 External stimulation of muscle rings
(A) Stimulation setup for optical pulse stimulation of bio-bots. (B) Representative optical pulse train signal. (C) Stimulation setup for electrical pulse stimulation of bio-bots. (D) Representative electrical biphasic pulse signal.
Supplementary Figure 3 Muscle ring exercise training regimen
Protocol for stimulating bio-bots using a static mechanical stimulus (imposed by tethering the bio-bot to an underlying glass coverslip) starting Day 1, immediately after ring transfer, and a dynamic optical stimulus (imposed by the apparatus shown in Supplementary Figure 1 starting Day 4, after transferring the bio-bots to differentiation medium.
Supplementary Figure 4 Modulus as a Function of Energy Dose
Plot of Young’s Modulus for PEGDA 700 g mol-1 as a function of the UV energy dose imposed by the laser of the SLA during fabrication.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 and Supplementary Methods 1–3. (PDF 879 kb)
Supplementary Data
CAD files of muscle ring and muscle strip injection molds and symmetric and asymmetric one-ring, two-ring, and four-ring bio-bot skeletons; and FEA template file of one-ring bio-bot skeleton for computational modeling. (ZIP 673 kb)
A modular approach to designing, fabricating, and controlling muscle-powered machines.
Overview of major steps in protocol, including CAD design, 3D printing, muscle seeding, muscle differentiation, and bio-bot functional assessment. (MP4 28823 kb)
Electrical stimulation of unconstrained muscle ring.
Electrical stimulation (1 Hz) controls contraction in a muscle ring that is untethered to a bio-bot skeleton. (MP4 2968 kb)
Directional locomotion in a bio-bot.
Optical stimulation (4 Hz) drives directional locomotion of a one-leg asymmetric bio-bot in the direction of the longer pillar. (MOV 20777 kb)
Rotational locomotion in a bio-bot.
Optical stimulation (2 Hz) of one half of one muscle ring in a two-leg symmetric bio-bot drives rotational locomotion. (MOV 29692 kb)
Rights and permissions
About this article
Cite this article
Raman, R., Cvetkovic, C. & Bashir, R. A modular approach to the design, fabrication, and characterization of muscle-powered biological machines. Nat Protoc 12, 519–533 (2017). https://doi.org/10.1038/nprot.2016.185
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2016.185
This article is cited by
-
Recent trends in bioartificial muscle engineering and their applications in cultured meat, biorobotic systems and biohybrid implants
Communications Biology (2022)
-
The emerging technology of biohybrid micro-robots: a review
Bio-Design and Manufacturing (2022)
-
Soft actuators for real-world applications
Nature Reviews Materials (2021)
-
Biomimetic approaches toward smart bio-hybrid systems
Nano Research (2018)
-
Investigating the Life Expectancy and Proteolytic Degradation of Engineered Skeletal Muscle Biological Machines
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.