Abstract
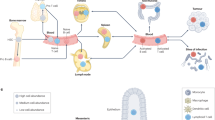
Induction of B-cell immunity against infection depends on the initiation of the germinal center (GC) reaction in secondary lymphoid organs. Ex vivo recapitulation of the GC reaction in 2D cultures results in transient cell growth, with poor yield and short-term survival. Furthermore, no reported 2D ex vivo system can modulate the kinetics of a GC-like phenotype or the rate of antibody class switching. This protocol describes a methodology for developing immune organoids that partially mimic the B-cell zone of a lymphoid tissue, for efficient and rapid generation of B cells with a GC-like phenotype from naive murine B cells. The organoid is composed of a bioadhesive protein, gelatin, that is transformed into an ionically cross-linked hydrated network using biocompatible silicate nanoparticles (SiNPs). We explain how to establish the immune organoid culture to sustain immune cell proliferation and transformation into a GC-like phenotype. Starting with cell encapsulation in digested lymphoid tissues, clusters of proliferating B cells with a GC-like phenotype can be generated in the organoids at controlled rates, within ∼1 week. The culture methodology described here is currently the only one that allows the accelerated induction of a GC-like phenotype in B cells and supports a controllable immunoglobulin class-switching reaction. This method can be easily implemented in a typical tissue culture room by personnel with standard mammalian cell culture expertise.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Shi, W. et al. Transcriptional profiling of mouse B cell terminal differentiation defines a signature for antibody-secreting plasma cells. Nat. Immunol. 16, 663–673 (2015).
Saylor, C., Dadachova, E. & Casadevall, A. Monoclonal antibody-based therapies for microbial diseases. Vaccine 27, G38–G46 (2009).
Sabbatino, F. et al. Novel tumor antigen-specific monoclonal antibody-based immunotherapy to eradicate both differentiated cancer cells and cancer-initiating cells in solid tumors. Semin. Oncol. 41, 685–699 (2014).
Weiner, L.M., Murray, J.C. & Shuptrine, C. Antibody-based immunotherapy of cancer. Cell 148, 1081–1084 (2012).
Caspi, R. Immunotherapy of autoimmunity and cancer: the penalty for success. Nat. Rev. Immunol. 8, 970–976 (2008).
Nutt, S.L., Hodgkin, P.D., Tarlinton, D.M. & Corcoran, L.M. The generation of antibody-secreting plasma cells. Nat. Rev. Immunol. 15, 160–171 (2015).
Schiemann, B. et al. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science 293, 2111–2114 (2001).
Heesters, B.A., Myers, R.C. & Carroll, M.C. Follicular dendritic cells: dynamic antigen libraries. Nat. Rev. Immunol. 14, 495–504 (2014).
Cerutti, A. et al. CD40 ligand and appropriate cytokines induce switching to IgG, IgA, and IgE and coordinated germinal center and plasmacytoid phenotypic differentiation in a human monoclonal IgM+ IgD+ B cell line. J. Immunol. 160, 2145–2157 (1998).
von Bergwelt-Baildon, M.S. et al. Human primary and memory cytotoxic T lymphocyte responses are efficiently induced by means of CD40-activated B cells as antigen-presenting cells: potential for clinical application. Blood 99, 3319–3325 (2002).
Schultze, J.L. et al. CD40-activated human B cells: an alternative source of highly efficient antigen presenting cells to generate autologous antigen-specific T cells for adoptive immunotherapy. J. Clin. Invest. 100, 2757 (1997).
Coughlin, C.M., Vance, B.A., Grupp, S.A. & Vonderheide, R.H. RNA-transfected CD40-activated B cells induce functional T-cell responses against viral and tumor antigen targets: implications for pediatric immunotherapy. Blood 103, 2046–2054 (2004).
Wang, X., Rodda, L.B., Bannard, O. & Cyster, J.G. Integrin-mediated interactions between B cells and follicular dendritic cells influence germinal center B cell fitness 192, 4601–4609 (2014).
Allen, C.D. & Cyster, J.G. Follicular dendritic cell networks of primary follicles and germinal centers: phenotype and function. Semin. Immunol. 20, 14–25 (2008).
Song, J. et al. Extracellular matrix of secondary lymphoid organs impacts on B-cell fate and survival. Proc. Natl. Acad. Sci. USA 110, E2915–E2924 (2013).
Carrasco, Y.R. & Batista, F.D. B-cell activation by membrane-bound antigens is facilitated by the interaction of VLA-4 with VCAM-1. EMBO J. 25, 889–899 (2006).
Liu, Y.J. et al. Mechanism of antigen-driven selection in germinal centres. Nature 342, 929–931 (1989).
Martinez-Valdez, H. et al. Human germinal center B cells express the apoptosis-inducing genes Fas, c-myc, P53, and Bax but not the survival gene bcl-2. J. Exp. Med. 183, 971–977 (1996).
Klein, U. & Dalla-Favera, R. Germinal centres: role in B-cell physiology and malignancy. Nat. Rev. Immunol. 8, 22–33 (2008).
Nojima, T. et al. In-vitro derived germinal centre B cells differentially generate memory B or plasma cells in vivo. Nat. Commun. 2, 465 (2011).
De Silva, N.S. & Klein, U. Dynamics of B cells in germinal centres. Nat. Rev. Immunol. 15, 137–148 (2015).
Shulman, Z. et al. T follicular helper cell dynamics in germinal centers. Science 341, 673–677 (2013).
Schwickert, T.A. et al. In vivo imaging of germinal centres reveals a dynamic open structure. Nature 446, 83–87 (2007).
Allen, C.D. et al. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat. Immunol. 5, 943–952 (2004).
Berek, C., Berger, A. & Apel, M. Maturation of the immune response in germinal centers. Cell 67, 1121–1129 (1991).
Pattengale, P., Smith, R. & Gerber, P. Selective transformation of B lymphocytes by EB virus. Lancet 302, 93–94 (1973).
Morello, F. et al. Differential gene expression of blood-derived cell lines in familial combined hyperlipidemia. Arterioscler. Thromb. Vasc. Biol. 24, 2149–2154 (2004).
Amoli, M., Carthy, D., Platt, H. & Ollier, W. EBV Immortalization of human B lymphocytes separated from small volumes of cryo-preserved whole blood. Int. J. Epidemiol. 37, i41–i45 (2008).
Traggiai, E. et al. An efficient method to make human monoclonal antibodies from memory B cells: potent neutralization of SARS coronavirus. Nat. Med. 10, 871–875 (2004).
Glasky, M.S. & Reading, C.L. Stability of specific immunoglobulin secretion by EBV-transformed lymphoblastoid cells and human-murine heterohybridomas. Hybridoma 8, 377–389 (1989).
Singh, A. & Peppas, N.A. Hydrogels and scaffolds for immunomodulation. Adv. Mat. 26, 6530–6541 (2014).
Purwada, A., Roy, K. & Singh, A. Engineering vaccines and niches for immune modulation. Acta Biomater. 10, 1728–1740 (2014).
Irvine, D.J., Stachowiak, A.N. & Hori, Y. Lymphoid tissue engineering: invoking lymphoid tissue neogenesis in immunotherapy and models of immunity. Sem. Immunol. 20, 137–146 (2008).
Suematsu, S. & Watanabe, T. Generation of a synthetic lymphoid tissue-like organoid in mice. Nat. Biotechnol. 22, 1539–1545 (2004).
Stachowiak, A.N. & Irvine, D.J. Inverse opal hydrogel-collagen composite scaffolds as a supportive microenvironment for immune cell migration. J. Biomed. Mater. Res. A 85, 815–828 (2008).
Gaharwar, A.K. et al. Shear-thinning nanocomposite hydrogels for the treatment of hemorrhage. ACS Nano 8, 9833–9842 (2014).
Xavier, J.R. et al. Bioactive nanoengineered hydrogels for bone tissue engineering: a growth-factor-free approach. ACS Nano 9, 3109–3118 (2015).
Victoratos, P. et al. FDC-specific functions of p55TNFR and IKK2 in the development of FDC networks and of antibody responses. Immunity 24, 65–77 (2006).
Goodnow, C.C., Vinuesa, C.G., Randall, K.L., Mackay, F. & Brink, R. Control systems and decision making for antibody production. Nat. Immunol. 11, 681–688 (2010).
Dumin, J.A. et al. Pro-collagenase-1 (matrix metalloproteinase-1) binds the alpha(2)beta(1) integrin upon release from keratinocytes migrating on type I collagen. J. Biol. Chem. 276, 29368–29374 (2001).
Purwada, A. et al. Ex vivo engineered immune organoids for controlled germinal center reactions. Biomaterials 63, 24–34 (2015).
Cerchietti, L.C. et al. BCL6 repression of EP300 in human diffuse large B cell lymphoma cells provides a basis for rational combinatorial therapy. J. Clin. Invest. 120, 4569–4582 (2010).
Cerchietti, L.C. et al. A small-molecule inhibitor of BCL6 kills DLBCL cells in vitro and in vivo. Cancer Cell 17, 400–411 (2010).
Ame-Thomas, P. et al. Characterization of intratumoral follicular helper T cells in follicular lymphoma: role in the survival of malignant B cells. Leukemia 26, 1053–1063 (2012).
Lenz, G. et al. Stromal gene signatures in large-B-cell lymphomas. N. Engl. J. Med. 359, 2313–2323 (2008).
Cayrol, F. et al. Integrin αvβ3 acting as membrane receptor for thyroid hormones mediates angiogenesis in malignant T cells. Blood 125, 841–851 (2015).
Kasturi, S.P. et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature 470, 543–547 (2011).
Chiarle, R. et al. The anaplastic lymphoma kinase is an effective oncoantigen for lymphoma vaccination. Nat. Med. 14, 676–680 (2008).
Davis, R.E. et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature 463, 88–92 (2010).
Hailfinger, S. et al. Essential role of MALT1 protease activity in activated B cell-like diffuse large B-cell lymphoma. Proc. Natl. Acad. Sci. USA 106, 19946–19951 (2009).
Lenz, G. et al. Oncogenic CARD11 mutations in human diffuse large B cell lymphoma. Science 319, 1676–1679 (2008).
Lenz, G. et al. Aberrant immunoglobulin class switch recombination and switch translocations in activated B cell-like diffuse large B cell lymphoma. J. Exp. Med. 204, 633–643 (2007).
Lenz, G. et al. Molecular subtypes of diffuse large B-cell lymphoma arise by distinct genetic pathways. Proc. Natl. Acad. Sci. USA 105, 13520–13525 (2008).
Nagel, D. et al. Pharmacologic inhibition of MALT1 protease by phenothiazines as a therapeutic approach for the treatment of aggressive ABC-DLBCL. Cancer Cell 22, 825–837 (2012).
Tian, Y.F. et al. Integrin-specific hydrogels as adaptable tumor organoids for malignant B and T cells. Biomaterials 73, 110–119 (2015).
Caganova, M. et al. Germinal center dysregulation by histone methyltransferase EZH2 promotes lymphomagenesis. J. Clin. Invest. 123, 5009–5022 (2013).
Béguelin, W. et al. EZH2 is required for germinal center formation and somatic EZH2 mutations promote lymphoid transformation. Cancer Cell 23, 677–692 (2013).
Su, I.H. et al. Ezh2 controls B cell development through histone H3 methylation and Igh rearrangement. Nat. Immunol. 4, 124–131 (2003).
Fontan, L. et al. MALT1 small molecule inhibitors specifically suppress ABC-DLBCL in vitro and in vivo. Cancer Cell 22, 812–824 (2012).
Clozel, T. et al. Mechanism-based epigenetic chemosensitization therapy of diffuse large B cell lymphoma. Cancer Discov. 3, 1002–1019 (2013).
Linterman, M.A. How T follicular helper cells and the germinal centre response change with age. Immunol. Cell Biol. 92, 72–79 (2014).
Purwada, A. et al. Self-assembly protein nanogels for safer cancer immunotherapy. Adv. Healthc. Mater. 5, 1413–1419 (2016).
Singh, A. et al. An injectable synthetic immune-priming center mediates efficient T-cell class switching and T-helper 1 response against B cell lymphoma. J. Control. Release 155, 184–192 (2011).
Singh, A., Suri, S. & Roy, K. In-situ crosslinking hydrogels for combinatorial delivery of chemokines and siRNA-DNA carrying microparticles to dendritic cells. Biomaterials 30, 5187–5200 (2009).
Singh, A. et al. Efficient modulation of T-cell response by dual-mode, single-carrier delivery of cytokine-targeted siRNA and DNA vaccine to antigen-presenting cells. Mol. Ther. 16, 2011–2021 (2008).
Moon, J.J. et al. Enhancing humoral responses to a malaria antigen with nanoparticle vaccines that expand Tfh cells and promote germinal center induction. Proc. Natl. Acad. Sci. USA 109, 1080–1085 (2012).
Look, M. et al. Nanogel-based delivery of mycophenolic acid ameliorates systemic lupus erythematosus in mice. J. Clin. Invest. 123, 1741–1749 (2013).
Zhou, P. et al. In vivo discovery of immunotherapy targets in the tumour microenvironment. Nature 506, 52–57 (2014).
Qin, H. et al. Vaccine site inflammation potentiates idiotype DNA vaccine-induced therapeutic T cell-, and not B cell-, dependent antilymphoma immunity. Blood 114, 4142–4149 (2009).
Chiarle, R. et al. Stat3 is required for ALK-mediated lymphomagenesis and provides a possible therapeutic target. Nat. Med. 11, 623–629 (2005).
Chiarle, R. et al. NPM-ALK transgenic mice spontaneously develop T-cell lymphomas and plasma cell tumors. Blood 101, 1919–1927 (2003).
Krzyzak, L. et al. CD83 modulates B cell activation and germinal center responses. J. Immunol. 196, 3581–3594 (2016).
Klein, U. et al. Transcriptional analysis of the B cell germinal center reaction. Proc. Natl. Acad. Sci. USA 100, 2639–2644 (2003).
Ortega-Molina, A. et al. The histone lysine methyltransferase KMT2D sustains a gene expression program that represses B cell lymphoma development. Nat. Med. 21, 1199–1208 (2015).
Gorbet, M.B. & Sefton, M.V. Endotoxin: the uninvited guest. Biomaterials 26, 6811–6817 (2005).
Round, J.L. et al. Scaffold protein Dlgh1 coordinates alternative p38 kinase activation, directing T cell receptor signals toward NFAT but not NF-kappaB transcription factors. Nat. Immunol. 8, 154–161 (2007).
Watarai, H., Nakagawa, R., Omori-Miyake, M., Dashtsoodol, N. & Taniguchi, M. Methods for detection, isolation and culture of mouse and human invariant NKT cells. Nat. Protoc. 3, 70–78 (2008).
Moutai, T., Yamana, H., Nojima, T. & Kitamura, D. A novel and effective cancer immunotherapy mouse model using antigen-specific B Cells selected in vitro. PloS One 9, e92732 (2014).
Vuong, B.Q. et al. Specific recruitment of protein kinase A to the immunoglobulin locus regulates class-switch recombination. Nat. Immunol. 10, 420–426 (2009).
Shinkura, R. et al. Separate domains of AID are required for somatic hypermutation and class-switch recombination. Nat. Immunol. 5, 707–712 (2004).
Liu, N., Ohnishi, N., Ni, L., Akira, S. & Bacon, K.B. CpG directly induces T-bet expression and inhibits IgG1 and IgE switching in B cells. Nat. Immunol. 4, 687–693 (2003).
Talay, O. et al. IgE(+) memory B cells and plasma cells generated through a germinal-center pathway. Nat. Immunol. 13, 396–404 (2012).
Fischer, A.H., Jacobson, K.A., Rose, J. & Zeller, R. Cutting sections of paraffin-embedded tissues. CSH Protoc. 2008 http://dx.doi.org/10.1101/pdb.prot4987 (2008).
Lee, T.T. et al. Light-triggered in vivo activation of adhesive peptides regulates cell adhesion, inflammation and vascularization of biomaterials. Nat. Mater. 14, 352–360 (2015).
Whitmire, R.E. et al. Self-assembling nanoparticles for intra-articular delivery of anti-inflammatory proteins. Biomaterials 33, 7665–7675 (2012).
Syrbu, S.I. & Cohen, M.B. An enhanced antigen-retrieval protocol for immunohistochemical staining of formalin-fixed, paraffin-embedded tissues. Methods Mol. Biol. 717, 101–110 (2011).
Acknowledgements
We acknowledge financial support from a National Science Foundation (NSF) CAREER Award (DMR-1554275 (to A.S.)) and the National Institutes of Health (1R21CA185236-01, to A.S.). We thank D. Kitamura at Tokyo University of Science for providing 40LB cells. We thank L. Cerchietti and his laboratory at Weill Cornell Medical College of Cornell University for providing histology imaging expertise and A. Gaharwar and his laboratory at Texas A&M University for silicate nanoparticle imaging expertise. All procedures were approved by Cornell University's Institutional Animal Care and Use Committee. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Author information
Authors and Affiliations
Contributions
A.P. and A.S. wrote the manuscript. A.P. conducted the experiments, and A.P. and A.S. performed data analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Ex vivo induction into CD83 phenotype within the immune organoid.
Naïve B cells with 40LB cells were co-encapsulated with the immune organoids. Left panel shows the histogram overlay of flow cytometry analysis indicating a shift in CD84 signal. Right panel indicates Median fluorescence intensity of CD19+CD83+ cells inside the organoids and comparative controls (*p<0.05, Analysis of variance (ANOVA), n=3, data represents Mean ± Standard Error)
Supplementary information
Supplementary Text and Figures
Supplementary Figure 1. (PDF 133 kb)
Rights and permissions
About this article
Cite this article
Purwada, A., Singh, A. Immuno-engineered organoids for regulating the kinetics of B-cell development and antibody production. Nat Protoc 12, 168–182 (2017). https://doi.org/10.1038/nprot.2016.157
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2016.157
This article is cited by
-
Tertiary lymphoid structural heterogeneity determines tumour immunity and prospects for clinical application
Molecular Cancer (2024)
-
Organoids in virology
npj Viruses (2024)
-
Characterizing influence of rCHOP treatment on diffuse large B-cell lymphoma microenvironment through in vitro microfluidic spheroid model
Cell Death & Disease (2024)
-
Collagen-based biomaterials in organoid technology for reproductive medicine: composition, characteristics, and applications
Collagen and Leather (2023)
-
Bioengineering translational models of lymphoid tissues
Nature Reviews Bioengineering (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.