Abstract
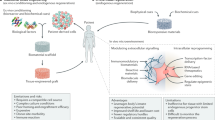
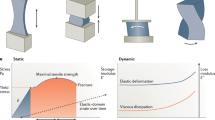
Tremendous progress has been achieved in the field of tissue engineering in the past decade. Several major challenges laid down 10 years ago, have been studied, including renewable cell sources, biomaterials with tunable properties, mitigation of host responses, and vascularization. Here we review advancements in these areas and envision directions of further development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Langer, R. & Vacanti, J.P. Tissue engineering. Science 260, 920–926 (1993).
Khademhosseini, A., Vacanti, J.P. & Langer, R. Progress in tissue engineering. Sci. Am. 300, 64–71 (2009).
Khademhosseini, A. & Langer, R. Microengineered hydrogels for tissue engineering. Biomaterials 28, 5087–5092 (2007).
Okita, K., Ichisaka, T. & Yamanaka, S. Generation of germline-competent induced pluripotent stem cells. Nature 448, 313–317 (2007).
Yu, J. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 (2007).
Engler, A.J., Sen, S., Sweeney, H.L. & Discher, D.E. Matrix elasticity directs stem cell lineage specification 126, 677–689 (2006).
DeForest, C.A., Polizzotti, B.D. & Anseth, K.S. Sequential click reactions for synthesizing and patterning three-dimensional cell microenvironments. Nat. Mater. 8, 659–664 (2009).
DeForest, C.A. & Anseth, K.S. Cytocompatible click-based hydrogels with dynamically tunable properties through orthogonal photoconjugation and photocleavage reactions. Nat. Chem. 3, 925–931 (2011).
Martino, M.M., Briquez, P.S., Ranga, A., Lutolf, M.P. & Hubbell, J.A. Heparin-binding domain of fibrin(ogen) binds growth factors and promotes tissue repair when incorporated within a synthetic matrix. Proc. Natl. Acad. Sci. USA 110, 4563–4568 (2013).
Pakulska, M.M., Miersch, S. & Shoichet, M.S. Designer protein delivery: From natural to engineered affinity-controlled release systems. Science 351, aac4750 (2016).
Veiseh, O. et al. Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat. Mater. 14, 643–651 (2015).
Vegas, A.J. et al. Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates. Nat. Biotechnol. 34, 345–352 (2016).
Langer, R. et al. Tissue engineering: biomedical applications. Tissue Eng. 1, 151–161 (1995).
Vacanti, J.P. & Langer, R. Tissue engineering: the design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet 354 (Suppl. 1): S32–S34 (1999).
Qi, H. et al. DNA-directed self-assembly of shape-controlled hydrogels. Nat. Commun. 4, 2275 (2013).
Todhunter, M.E. et al. Programmed synthesis of three-dimensional tissues. Nat. Methods 12, 975–981 (2015).
Cohen, D.L., Malone, E., Lipson, H. & Bonassar, L.J. Direct freeform fabrication of seeded hydrogels in arbitrary geometries. Tissue Eng. 12, 1325–1335 (2006).
Khalil, S., Nam, J. & Sun, W. Multi-nozzle deposition for construction of 3d biopolymer tissue scaffolds. Rapid Prototyping J. 11, 9–17 (2005).
Miller, J.S. et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 11, 768–774 (2012).
Kolesky, D.B. et al. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv. Mater. 26, 3124–3130 (2014).
Murphy, S.V. & Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 32, 773–785 (2014).
Colosi, C. et al. Microfluidic bioprinting of heterogeneous 3D tissue constructs using low-viscosity bioink. Adv. Mater. 28, 677–684 (2016).
Ober, T.J., Foresti, D. & Lewis, J.A. Active mixing of complex fluids at the microscale. Proc. Natl. Acad. Sci. USA 112, 12293–12298 (2015).
Kang, H.-W. et al. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 34, 312–319 (2016).
Karp, J.M. & Leng Teo, G.S. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell 4, 206–216 (2009).
Ranganath, S.H., Levy, O., Inamdar, M.S. & Karp, J.M. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell 10, 244–258 (2012).
Sarkar, D. et al. Engineered cell homing. Blood 118, e184–e191 (2011).
Levy, O. et al. mRNA-engineered mesenchymal stem cells for targeted delivery of interleukin-10 to sites of inflammation. Blood 122, e23–e32 (2013).
Zuk, P.A. et al. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 13, 4279–4295 (2002).
De Coppi, P. et al. Isolation of amniotic stem cell lines with potential for therapy. Nat. Biotechnol. 25, 100–106 (2007).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013).
Ran, F.A. et al. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8, 2281–2308 (2013).
Wang, H. et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 153, 910–918 (2013).
Servick, K. Gene-editing method revives hopes for transplanting pig organs into people. Science 10.1126/science.aad4700 (2015).
Reardon, S. New life for pig-to-human transplants. Nature 527, 152–154 (2015).
Reardon, S. Gene-editing record smashed in pigs. Nature 10.1038/nature.2015.18525 (2015).
Azagarsamy, M.A. & Anseth, K.S. Bioorthogonal click chemistry: An indispensable tool to create multifaceted cell culture scaffolds. ACS Macro Lett. 2, 5–9 (2013).
Sakiyama-Elbert, S.E. & Hubbell, J.A. Controlled release of nerve growth factor from a heparin-containing fibrin-based cell ingrowth matrix. J. Control. Release 69, 149–158 (2000).
Sakiyama-Elbert, S.E. & Hubbell, J.A. Development of fibrin derivatives for controlled release of heparin-binding growth factors. J. Control. Release 65, 389–402 (2000).
Swift, J. et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 341, 1240104 (2013).
Huebsch, N. et al. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat. Mater. 9, 518–526 (2010).
Khetan, S. et al. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat. Mater. 12, 458–465 (2013).
Yang, C., Tibbitt, M.W., Basta, L. & Anseth, K.S. Mechanical memory and dosing influence stem cell fate. Nat. Mater. 13, 645–652 (2014).
Anderson, J.M., Rodriguez, A. & Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 20, 86–100 (2008).
Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 29, 2941–2953 (2008).
Mantovani, A. et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25, 677–686 (2004).
Porcheray, F. et al. Macrophage activation switching: an asset for the resolution of inflammation. Clin. Exp. Immunol. 142, 481–489 (2005).
Fishman, J.M. et al. Immunomodulatory effect of a decellularized skeletal muscle scaffold in a discordant xenotransplantation model. Proc. Natl. Acad. Sci. USA 110, 14360–14365 (2013).
Spiller, K.L. et al. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials 35, 4477–4488 (2014).
Spiller, K.L. et al. Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials 37, 194–207 (2015).
Brown, B.N., Ratner, B.D., Goodman, S.B., Amar, S. & Badylak, S.F. Macrophage polarization: an opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials 33, 3792–3802 (2012).
Mokarram, N. & Bellamkonda, R.V. A perspective on immunomodulation and tissue repair. Ann. Biomed. Eng. 42, 338–351 (2014).
Du, Y., Lo, E., Ali, S. & Khademhosseini, A. Directed assembly of cell-laden microgels for fabrication of 3D tissue constructs. Proc. Natl. Acad. Sci. USA 105, 9522–9527 (2008).
Du, Y. et al. Surface-directed assembly of cell-laden microgels. Biotechnol. Bioeng. 105, 655–662 (2010).
Ke, Y., Ong, L.L., Shih, W.M. & Yin, P. Three-dimensional structures self-assembled from DNA bricks. Science 338, 1177–1183 (2012).
Wei, B., Dai, M. & Yin, P. Complex shapes self-assembled from single-stranded DNA tiles. Nature 485, 623–626 (2012).
Park, A., Wu, B. & Griffith, L.G. Integration of surface modification and 3D fabrication techniques to prepare patterned poly(L-lactide) substrates allowing regionally selective cell adhesion. J. Biomater. Sci. Polym. Ed. 9, 89–110 (1998).
Giordano, R.A. et al. Mechanical properties of dense polylactic acid structures fabricated by three dimensional printing. J. Biomater. Sci. Polym. Ed. 8, 63–75 (1996).
Vozzi, G., Flaim, C., Ahluwalia, A. & Bhatia, S. Fabrication of PLGA scaffolds using soft lithography and microsyringe deposition. Biomaterials 24, 2533–2540 (2003).
Wilson, W.C. Jr. & Boland, T. Cell and organ printing 1: protein and cell printers. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 272, 491–496 (2003).
Boland, T., Mironov, V., Gutowska, A., Roth, E.A. & Markwald, R.R. Cell and organ printing 2: fusion of cell aggregates in three-dimensional gels. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 272, 497–502 (2003).
Malda, J. et al. 25th anniversary article: Engineering hydrogels for biofabrication. Adv. Mater. 25, 5011–5028 (2013).
Bertassoni, L.E. et al. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip 14, 2202–2211 (2014).
Lee, V.K. et al. Creating perfused functional vascular channels using 3D bio-printing technology. Biomaterials 35, 8092–8102 (2014).
Kolesky, D.B., Homan, K.A., Skylar-Scott, M.A. & Lewis, J.A. Three-dimensional bioprinting of thick vascularized tissues. Proc. Natl. Acad. Sci. USA 113, 3179–3184 (2016).
Bhattacharjee, T. et al. Writing in the granular gel medium. Sci. Adv. 1, e1500655 (2015).
Christensen, K. et al. Freeform inkjet printing of cellular structures with bifurcations. Biotechnol. Bioeng. 112, 1047–1055 (2015).
Highley, C.B., Rodell, C.B. & Burdick, J.A. Direct 3d printing of shear-thinning hydrogels into self-healing hydrogels. Adv. Mater. 27, 5075–5079 (2015).
Shim, J.-H., Lee, J.-S., Kim, J.Y. & Cho, D.-W.Bioprintingof a mechanically enhanced three-dimensional dual cell-laden construct for osteochondral tissue engineering using a multi-head tissue/organ building system.. J. Micromech. Microeng. 22, 085014 (2012).
Tibbits, S. 4D printing: multi-material shape change. Architectural Design 84, 116–121 (2014).
Sydney Gladman, A., Matsumoto, E.A., Nuzzo, R.G., Mahadevan, L. & Lewis, J.A. Biomimetic 4D printing. Nat. Mater. 15, 413–418 (2016).
Badylak, S.F. The extracellular matrix as a scaffold for tissue reconstruction. in Seminars in Cell & Developmental Biology 377–383 (Elsevier, 2002).
Ott, H.C. et al. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat. Med. 14, 213–221 (2008).
Badylak, S.F., Taylor, D. & Uygun, K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu. Rev. Biomed. Eng. 13, 27–53 (2011).
Song, J.J. & Ott, H.C. Organ engineering based on decellularized matrix scaffolds. Trends Mol. Med. 17, 424–432 (2011).
Arenas-Herrera, J.E., Ko, I.K., Atala, A. & Yoo, J.J. Decellularization for whole organ bioengineering. Biomed. Mater. 8, 014106 (2013).
Kaushal, S. et al. Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat. Med. 7, 1035–1040 (2001).
Amiel, G.E. et al. Engineering of blood vessels from acellular collagen matrices coated with human endothelial cells. Tissue Eng. 12, 2355–2365 (2006).
Zhang, W. et al. Elastomeric free-form blood vessels for interconnecting organs on chip systems. Lab Chip 16, 1579–1586 (2016).
Lu, T.-Y. et al. Repopulation of decellularized mouse heart with human induced pluripotent stem cell-derived cardiovascular progenitor cells. Nat. Commun. 4, 2307 (2013).
Ott, H.C. et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nat. Med. 16, 927–933 (2010).
Petersen, T.H. et al. Tissue-engineered lungs for in vivo implantation. Science 329, 538–541 (2010).
Uygun, B.E. et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat. Med. 16, 814–820 (2010).
Baptista, P.M. et al. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology 53, 604–617 (2011).
Sullivan, D.C. et al. Decellularization methods of porcine kidneys for whole organ engineering using a high-throughput system. Biomaterials 33, 7756–7764 (2012).
Song, J.J. et al. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat. Med. 19, 646–651 (2013).
Atala, A., Bauer, S.B., Soker, S., Yoo, J.J. & Retik, A.B. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet 367, 1241–1246 (2006).
Goh, S.-K. et al. Perfusion-decellularized pancreas as a natural 3D scaffold for pancreatic tissue and whole organ engineering. Biomaterials 34, 6760–6772 (2013).
Teng, Y.D. et al. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc. Natl. Acad. Sci. USA 99, 3024–3029 (2002).
Niklason, L.E. et al. Functional arteries grown in vitro. Science 284, 489–493 (1999).
Lancaster, M.A. et al. Cerebral organoids model human brain development and microcephaly. Nature 501, 373–379 (2013).
Choi, S.H. et al. A three-dimensional human neural cell culture model of Alzheimer's disease. Nature 515, 274–278 (2014).
Huh, D., Hamilton, G.A. & Ingber, D.E. From 3D cell culture to organs-on-chips. Trends Cell Biol. 21, 745–754 (2011).
Moraes, C., Mehta, G., Lesher-Perez, S.C. & Takayama, S. Organs-on-a-chip: a focus on compartmentalized microdevices. Ann. Biomed. Eng. 40, 1211–1227 (2012).
Wikswo, J.P. The relevance and potential roles of microphysiological systems in biology and medicine. Exp. Biol. Med. 239, 1061–1072 (2014).
Bhatia, S.N. & Ingber, D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 32, 760–772 (2014).
Bhise, N.S. et al. Organ-on-a-chip platforms for studying drug delivery systems. J. Control. Release 190, 82–93 (2014).
Zhang, Y.S. & Khademhosseini, A. Seeking the right context for evaluating nanomedicine: from tissue models in petri dishes to microfluidic organs-on-a-chip. Nanomedicine (Lond.) 10, 685–688 (2015).
Esch, E.W., Bahinski, A. & Huh, D. Organs-on-chips at the frontiers of drug discovery. Nat. Rev. Drug Discov. 14, 248–260 (2015).
Ingber, D.E. Reverse engineering human pathophysiology with organs-on-chips. Cell 164, 1105–1109 (2016).
Ebrahimkhani, M.R., Neiman, J.A., Raredon, M.S.B., Hughes, D.J. & Griffith, L.G. Bioreactor technologies to support liver function in vitro. Adv. Drug Deliv. Rev. 69-70, 132–157 (2014).
Huh, D. et al. Acoustically detectable cellular-level lung injury induced by fluid mechanical stresses in microfluidic airway systems. Proc. Natl. Acad. Sci. USA 104, 18886–18891 (2007).
Huh, D. et al. Reconstituting organ-level lung functions on a chip. Science 328, 1662–1668 (2010).
Wilmer, M.J. et al. Kidney-on-a-chip technology for drug-induced nephrotoxicity screening. Trends Biotechnol. 34, 156–170 (2016).
Kim, S., Lee, H., Chung, M. & Jeon, N.L. Engineering of functional, perfusable 3D microvascular networks on a chip. Lab Chip 13, 1489–1500 (2013).
Kim, H.J., Huh, D., Hamilton, G. & Ingber, D.E. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip 12, 2165–2174 (2012).
Torisawa, Y.-S. et al. Bone marrow-on-a-chip replicates hematopoietic niche physiology in vitro. Nat. Methods 11, 663–669 (2014).
Nawroth, J.C. et al. A tissue-engineered jellyfish with biomimetic propulsion. Nat. Biotechnol. 30, 792–797 (2012).
Shin, S.R. et al. Aligned carbon nanotube-based flexible gel substrates for engineering bio-hybrid tissue actuators. Adv. Funct. Mater. 25, 4486–4495 (2015).
Raman, R. et al. Optogenetic skeletal muscle-powered adaptive biological machines. Proc. Natl. Acad. Sci. USA 113, 3497–3502 (2016).
Menze, M.A. et al. Metabolic preconditioning of cells with AICAR-riboside: improved cryopreservation and cell-type specific impacts on energetics and proliferation. Cryobiology 61, 79–88 (2010).
Heo, Y.S. et al. “Universal” vitrification of cells by ultra-fast cooling. Technology (Singap. World Sci.) 3, 64–71 (2015).
Bruinsma, B.G. et al. Supercooling preservation and transplantation of the rat liver. Nat. Protoc. 10, 484–494 (2015).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
R.L. has equity ownership in In Vivo Therapeutics, Humacyte and Sigilon.
Rights and permissions
About this article
Cite this article
Khademhosseini, A., Langer, R. A decade of progress in tissue engineering. Nat Protoc 11, 1775–1781 (2016). https://doi.org/10.1038/nprot.2016.123
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2016.123
This article is cited by
-
Application of Biodegradable Bone Scaffolds Based on Poly(Lactic Acid) / Poly(Glycerol Succinic Acid) Containing Nano-Hydroxyapatite
Journal of Polymers and the Environment (2024)
-
Self-healing hydrogel as an injectable implant: translation in brain diseases
Journal of Biomedical Science (2023)
-
Fabrication of conductive hybrid scaffold based on polyaniline/polyvinyl alcohol–chitosan nanoparticles for skin tissue engineering application
Polymer Bulletin (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.