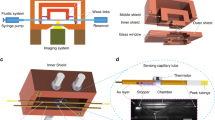
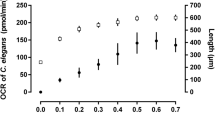
Abstract
Mitochondrial dysfunction is at the core of many diseases ranging from inherited metabolic diseases to common conditions that are associated with aging. Although associations between aging and mitochondrial function have been identified using mammalian models, much of the mechanistic insight has emerged from Caenorhabditis elegans. Mitochondrial respiration is recognized as an indicator of mitochondrial health. The Seahorse XF96 respirometer represents the state-of-the-art platform for assessing respiration in cells, and we adapted the technique for applications involving C. elegans. Here we provide a detailed protocol to optimize and measure respiration in C. elegans with the XF96 respirometer, including the interpretation of parameters and results. The protocol takes ∼2 d to complete, excluding the time spent culturing C. elegans, and it includes (i) the preparation of C. elegans samples, (ii) selection and loading of compounds to be injected, (iii) preparation and execution of a run with the XF96 respirometer and (iv) postexperimental data analysis, including normalization. In addition, we compare our XF96 application with other existing techniques, including the eight-well Seahorse XFp. The main benefits of the XF96 include the limited number of worms required and the high throughput capacity due to the 96-well format.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout












Similar content being viewed by others
References
Perry, C.G.R., Kane, D.A., Lanza, I.R. & Neufer, P.D. Methods for assessing mitochondrial function in diabetes. Diabetes 62, 1041–1053 (2013).
Hatefi, Y. The mitochondrial electron transport and oxidative phosphorylation system. Annu. Rev. Biochem. 54, 1015–1069 (1985).
Brand, M.D. & Nicholls, D.G. Assessing mitochondrial dysfunction in cells. Biochem. J. 435, 297–312 (2011).
Goldenthal, M.J. & Marín-García, J. Mitochondrial signaling pathways: a receiver/integrator organelle. Mol. Cell Biochem. 262, 1–16 (2004).
Galluzzi, L., Kepp, O. & Kroemer, G. Mitochondria: master regulators of danger signalling. Nat. Rev. Mol. Cell Biol. 13, 780–788 (2012).
Rizzuto, R., De Stefani, D., Raffaello, A. & Mammucari, C. Mitochondria as sensors and regulators of calcium signalling. Nat. Rev. Mol. Cell Biol. 13, 566–578 (2012).
Fernyhough, P., Roy Chowdhury, S.K. & Schmidt, R.E. Mitochondrial stress and the pathogenesis of diabetic neuropathy. Expert Rev. Endocrinol. Metab. 5, 39–49 (2010).
Ferreira, I.L., Resende, R., Ferreiro, E., Rego, A.C. & Pereira, C.F. Multiple defects in energy metabolism in Alzheimer's disease. Curr. Drug Targets 11, 1193–1206 (2010).
Jarrett, S.F., Lewin, A.S. & Boulton, M.E. The importance of mitochondria in age-related and inherited eye disorders. Opthalmic Res. 44, 179–190 (2010).
Kawamata, H. & Manfredi, G. Mitochondrial dysfunction and intracellular calcium dysregulation in ALS. Mech. Ageing Dev. 131, 517–526 (2010).
Ren, J., Pulakat, L., Whaley-Connell, A. & Sowers, J.R. Mitochondrial biogenesis in the metabolic syndrome and cardiovascular disease. J. Mol. Med. 88, 993–1001 (2010).
Rosenstock, T.R., Duarte, A.I. & Rego, A.C. Mitochondrial-associated metabolic changes and neurodegeneration in Huntington's disease – from clinical features to the bench. Curr. Drug Targets 11, 1218–1236 (2010).
Vafai, S.B. & Mootha, V.K. Mitochondrial disorders as windows into an ancient organelle. Nature 491, 374–383 (2012).
Andreux, P.A., Houtkooper, R.H. & Auwerx, J. Pharmacological approaches to restore mitochondrial function. Nat. Rev. Drug Discov. 12, 465–483 (2013).
Andreux, P.A. et al. A method to identify and validate mitochondrial modulators using mammalian cells and the worm C. elegans. Sci. Rep. 4, 05285 (2014).
Felkai, S. et al. CLK-1 controls respiration, behaviour and aging in the nematode Caenorhabditis elegans. EMBO J. 18, 1783–1792 (1999).
Kamo, N., Muratsugu, M., Hongoh, R. & Kobatake, Y. Membrane potential of mitochondria measured with an electrode sensitive to tetraphenyl phosphonium and relationship between proton electrochemical potential and phosphorylation potential in steady state. J. Membr. Biol. 49, 105–121 (1979).
Drew, B. & Leeuwenburgh, C. Method for measuring ATP production in isolated mitochondria: ATP production in brain and liver mitochondria of Fischer-344 rats with age and caloric restriction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 285, R1259–R1267 (2003).
Pan, X. et al. The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat. Cell Biol. 15, 1464–1472 (2013).
Chance, B. & Williams, G.R. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J. Biol. Chem. 217, 383–393 (1955).
Wu, M. et al. Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetics function and enhanced glycolysis dependency in human tumor cells. Am. J. Physiol. Cell Physiol. 292, C125–C136 (2007).
Gerencser, A.A. et al. Quantitative microplate-based respirometry with correction for oxygen diffusion. Anal. Chem. 81, 6868–6878 (2009).
Will, Y., Hynes, J., Ogurtsov, V.I. & Papkovsky, D.B. Analysis of mitochondrial function using phosphorescent oxygen-sensitive probes. Nat. Protoc. 1, 2563–2572 (2006).
Nadanaciva, S. et al. Assessment of drug-induced mitochondrial dysfunction via altered cellular respiration and acidification measured in 96-well platform. J. Bioenerg. Biomembr. 44, 421–437 (2012).
Yamamoto, H. et al. NCoR1 is a conserved physiological modulator of muscle mass and oxidative function. Cell 147, 827–839 (2011).
Houtkooper, R.H. et al. Mitonuclear protein imbalance as a conserved longevity mechanism. Nature 497, 451–457 (2013).
Mouchiroud, L. et al. The NAD+/sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell 154, 430–441 (2013).
Luz, A.L. et al. Mitochondrial morphology and fundamental parameters of the mitochondrial respiratory chain are altered in Caenorhabditis elegans strains deficient in mitochondrial dynamics and homeostasis processes. PLoS One 10, e0130940 (2015).
Lee, S.S. et al. A systematic RNAi screen identifies a critical role for mitochondria in C. elegans longevity. Nat. Genet. 33, 40–48 (2002).
Fire, A.Z. Gene silencing by double-stranded RNA. Cell Death Differ. 14, 1998–2012 (2007).
Grishok, A., Tabara, H. & Mello, C.C. Genetic requirements for inheritance of RNAi in C. elegans. Science 287, 2494–2497 (2000).
Dickinson, D.J., Ward, J.D., Reiner, D.J. & Goldstein, B. Engineering the Caenorhabditis elegans genome using Cas9-triggered homologous recombination. Nat. Methods 10, 1028–1034 (2013).
Wadsworth, W.G. & Riddle, D.L. Developmental regulation of energy metabolism in Caenorhabditis elegans. Dev. Biol. 132, 167–173 (1989).
Li, J. et al. Proteomics analysis of mitochondria from Caenorhabditis elegans. Proteomics 9, 4539–4554 (2009).
Mullaney, B.C. & Ashrafi, K. C. elegans fat storage and metabolic regulation. Biochim. Biophys. Acta 1791, 474–478 (2009).
Dancy, B.M., Sedensky, M.M. & Morgan, P.G. Mitochondrial bioenergetics and disease in Caenorhabditis elegans. Front Biosci. (Landmark Ed.) 20, 198–228 (2015).
Hill, B.G., Dranka, B.P., Zou, L., Chatham, J.C. & Darley-Usmar, V.M. Importance of the bioenergetics reserve capacity in response to cardiomyocyte stress induced by 4-hydroxynonenal. Biochem. J. 424, 99–107 (2009).
Dranka, B.P. et al. Assessing bioenergetic function in response to oxidative stress by metabolic profiling. Free. Radic. Biol. Med. 51, 1621–1635 (2011).
Sansbury, B.E., Jones, S.P., Riggs, D.W., Darley-Usmar, V.M. & Hill, B.G. Bioenergetic function in cardiovascular cells: the importance of the reserve capacity and its biological regulation. Chem. Biol. Interact. 191, 288–295 (2011).
Readnower, R.D., Brainard, R.E., Hill, B.G. & Jones, S.P. Standardized bioenergetics profiling of adult mouse cardiomyocytes. Physiol. Genomics 44, 1208–1213 (2012).
Wilson, D.F., Vinogradov, S., Lo, L.W. & Huang, L. Oxygen dependent quenching of phosphorescence: a status report. Adv. Exp. Med. Biol. 388, 101–107 (1996).
Schulz, T.J. et al. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 6, 280–293 (2007).
Ehrismann, D. et al. Studies on the activity of the hypoxia-inducible-factor hydroxylases using an oxygen consumption assay. Biochem. J. 401, 227–234 (2007).
Rand, J.B. & Johnson, C.D. Genetic pharmacology: interactions between drugs and gene products in Caenorhabditis elegans. Methods Cell Biol. 48, 187–204 (1995).
Burns, A.R. et al. A predictive model for drug bioaccumulation and bioactivity in Caenorhabditis elegans. Nat. Chem. Biol. 6, 549–557 (2010).
Zheng, S.-Q., Ding, A.-J., Li, G.-P., Wu, G.-S. & Luo, H.-R. Drug absorption efficiency in Caenorhabditis elegans delivered by different methods. PLoS One 8, e56877 (2013).
Massie, M.R., Lapoczka, E.M., Boggs, K.D., Stine, K.E. & White, G.E. Exposure to the metabolic inhibitor sodium azide induces stress protein expression and thermotolerance in the nematode Caenorhabditis elegans. Cell Stress Chaperones 8, 1–7 (2003).
Lanza, I.R. & Nair, K.S. Functional assessment of isolated mitochondria in vivo. Methods Enzymol. 457, 349–372 (2009).
Salabei, J.K., Gibb, A.A. & Hill, B.G. Comprehensive measurement of respiratory activity in permeabilized cells using extracellular flux analysis. Nat. Protoc. 9, 421–438 (2014).
Piper, H.M. et al. Development of ischemia-induced damage in defined mitochondrial subpopulations. J. Mol. Cell Cardio. 17, 885–896 (1985).
Picard, M. et al. Mitochondrial functional impairment with aging is exaggerated in isolated mitochondria compared to permeabilized myofibers. Aging Cell 9, 1032–1046 (2010).
Picard, M. et al. Mitochondrial structure and function are disrupted by standard isolation methods. PLoS One 6, e18317 (2011).
Picard, M., Taivassalo, T., Gouspillou, G. & Hepple, R.T. Mitochondria: isolation, structure and function. J. Physiol. 598, 4413–4421 (2011).
Kayser, E.B., Morgan, P.G., Hoppel, C.L. & Sedensky, M.M. Mitochondrial expression and function of GAS-1 in Caenorhabditis elegans. J. Biol. Chem. 276, 20551–20558 (2001).
Rivera-Ingraham, G.A., Bickmeyer, U. & Abele, D. The physiological response of the marine platyhelminth Macrostomum ligano to different environmental oxygen concentrations. J. Exp. Biol. 216, 2741–2751 (2013).
Steele, S.L., Prykhozhij, S.V. & Berman, J.N. Zebrafish as a model system for mitochondrial biology and diseases. Transl. Res. 163, 79–98 (2014).
Gibert, Y., McGee, S.L. & Ward, A.C. Metabolic profile analysis of zebrafish embryos. J. Vis. Exp. 14, e4300 (2013).
Hartman, N. et al. Mitochondrial DNA copy number and function decrease with age in the short-lived fish Nothobranchius furzeri. Aging Cell 10, 824–831 (2011).
Porta-de-la-Riva, M., Fontrodona, L., Villanueva, A. & Cerón, J. Basic Caenorhabditis elegans methods: synchronization and observation. J. Vis. Exp. 10, e4019 (2012).
Mitchell, D.H., Stiles, J.W., Santelli, J. & Sanadi, D.R. Synchronous growth and aging of Caenorhabditis elegans in the presence of fluorodeoxyuridine. J. Gerontol. 34, 28–36 (1979).
Davies, S.K., Leroi, A.M. & Bundy, J.G. Fluorodeoxyuridine affects the identification of metabolic responses to daf-2 status in Caenorhabditis elegans. Mech. Ageing Dev. 133, 46–49 (2012).
Rooney, J.P. et al. Effects of 5'-fluoro-2-deoxyuridine on mitochondrial biology in Caenorhabditis elegans. Exp. Gerontol. 56, 69–76 (2014).
Gruber, J., Ng, L.F., Poovathingsal, S.K. & Halliwell, B. Deceptively simple but simply deceptive – Caenorhabditis elegans lifespan studies: consideration for aging and antioxidant effects. FEBS Lett. 583, 3377–3387 (2009).
Boyd, W.A. et al. A high-throughput method for assessing chemical toxicity using a Caenorhabditis elegans reproduction assay. Toxicol. Appl. Pharmacol. 245, 153–159 (2010).
Zubovych, I.O., Straud, S. & Roth, M.G. Mitochondrial dysfunction confers resistance to multiple drugs in Caenorhabditis elegans. Mol. Biol. Cell 21, 956–969 (2010).
Wu, Y. et al. Multilayered genetic and omics dissection of mitochondrial activity in a mouse reference population. Cell 158, 1415–1430 (2014).
Weimer, S. et al. D-glucosamine supplementation extends life span of nematodes and ageing mice. Nat. Commun. 5, 3563 (2014).
Partridge, F.A., Tearle, A.W., Gravato-Nobre, M.J., Schafer, W.R. & Hodgkin, J. The C. elegans glycosyltransferase BUS-8 has two distinct and essential roles in epidermal morphogenesis. Dev. Biol. 317, 549–559 (2008).
MacVicar, T.D. & Lane, J.D. Impaired OMA1-dependent cleavage of OPA1 and reduced DRP1 fission activity combine to prevent mitophagy in cells that are dependent on oxidative phosphorylation. J. Cell Sci. 127, 2313–2325 (2014).
Fraser, A. et al. Functional genomic analysis of C. elegans chromosome I by systematic RNA interference. Nature 408, 325–330 (2000).
Kamath, R.S. et al. Systematic functional analysis of the C. elegans genome using RNAi. Nature 421, 231–237 (2003).
Stiernagle, T. Maintenance of C. elegans. in WormBook (ed. The C. elegans Research Community) doi:10.1895/wormbook.1.101.1 (2006).
Dancy, B.M. et al. Glutathione S-transferase mediates an ageing response to mitochondrial dysfunction. Mech. Ageing Dev. 153, 14–21 (2015).
Acknowledgements
We thank T. Ackermann for advice and C.F. Calkhoven for allowing us to make use of the Seahorse XF96 machine. Work in the Nollen group is financially supported by an European Research Council (ERC) starting grant (no. 281622). Work in the Houtkooper group is financially supported by an ERC starting grant (no. 638290) and a VIDI grant from ZonMw (no. 91715305). The Seahorse XF96 at the Academic Medical Center, Amsterdam, the Netherlands, was supported by a grant from NWO-Middelgroot (no. 91112009).
Author information
Authors and Affiliations
Contributions
M.K. performed most of the experiments, optimized the final experimental pipeline and wrote the manuscript together with R.H.H., and with contributions from all authors. H.M. and R.K performed experiments using the XFp/XF96 respirometers. L.M., R.H.H., B.M.D. and J.A. pioneered the use of XF respirometers with C. elegans and developed the initial protocols. M.K., H.M., E.A.A.N., B.M.D. and R.H.H. have been extensively involved in discussions and interpretations of results.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Titration of antimycin A and rotenone
(a) Titration of Antimycin A, n = 8-12 wells, and (b) Antimycin A & Rotenone, 8-16 wells, does not decrease the OCR. One-way ANOVA (ns). Bars are mean ± SEM.
Supplementary Figure 2 Respiration rate of E.coli.
Respiration rates of 15 worms compared to E. coli measured with a XFp respirometer. The respiration of E. coli is clearly lower than the respiration of worms. Bars are mean ± SEM.
Supplementary Figure 3 XF96 plate layout
This figure shows a representative plate layout that can be filled in for pre- and post-experimental preparation and analysis.
Supplementary Figure 4 Respiration correlates with the number of worms
Linear regression of the OCR as dependent variable of the number of worms in (a) L4s, (b) Adult day 3, (c) Adult day 5 and (d) Adult day 8. Grey area shows the confidence interval (95%). The dotted line in a-d shows the linear regression, n = 44-48 in a-d.
Supplementary information
Supplementary Information
Supplementary Figures 1–4 (PDF 542 kb)
Rights and permissions
About this article
Cite this article
Koopman, M., Michels, H., Dancy, B. et al. A screening-based platform for the assessment of cellular respiration in Caenorhabditis elegans. Nat Protoc 11, 1798–1816 (2016). https://doi.org/10.1038/nprot.2016.106
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2016.106
This article is cited by
-
A naturally occurring polyacetylene isolated from carrots promotes health and delays signatures of aging
Nature Communications (2023)
-
Early life changes in histone landscape protect against age-associated amyloid toxicities through HSF-1-dependent regulation of lipid metabolism
Nature Aging (2023)
-
Effects of colonization-associated gene yqiC on global transcriptome, cellular respiration, and oxidative stress in Salmonella Typhimurium
Journal of Biomedical Science (2022)
-
Measurement of oxygen consumption rates of human renal proximal tubule cells in an array of organ-on-chip devices to monitor drug-induced metabolic shifts
Microsystems & Nanoengineering (2022)
-
The sex-specific metabolic signature of C57BL/6NRj mice during aging
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.