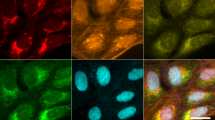
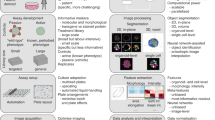
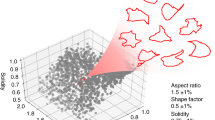
Abstract
In morphological profiling, quantitative data are extracted from microscopy images of cells to identify biologically relevant similarities and differences among samples based on these profiles. This protocol describes the design and execution of experiments using Cell Painting, which is a morphological profiling assay that multiplexes six fluorescent dyes, imaged in five channels, to reveal eight broadly relevant cellular components or organelles. Cells are plated in multiwell plates, perturbed with the treatments to be tested, stained, fixed, and imaged on a high-throughput microscope. Next, an automated image analysis software identifies individual cells and measures ∼1,500 morphological features (various measures of size, shape, texture, intensity, and so on) to produce a rich profile that is suitable for the detection of subtle phenotypes. Profiles of cell populations treated with different experimental perturbations can be compared to suit many goals, such as identifying the phenotypic impact of chemical or genetic perturbations, grouping compounds and/or genes into functional pathways, and identifying signatures of disease. Cell culture and image acquisition takes 2 weeks; feature extraction and data analysis take an additional 1–2 weeks.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Swinney, D.C. & Anthony, J. How were new medicines discovered? Nat. Rev. Drug Discov. 10, 507–519 (2011).
Swinney, D.C. The contribution of mechanistic understanding to phenotypic screening for first-in-class medicines. J. Biomol. Screen. 18, 1186–1192 (2013).
Moffat, J.G., Joachim, R. & David, B. Phenotypic screening in cancer drug discovery — past, present and future. Nat. Rev. Drug Discov. 13, 588–602 (2014).
Johannessen, C.M., Clemons, P.A. & Wagner, B.K. Integrating phenotypic small-molecule profiling and human genetics: the next phase in drug discovery. Trends Genet. 31, 16–23 (2015).
Bickle, M. The beautiful cell: high-content screening in drug discovery. Anal. Bioanal. Chem. 398, 219–226 (2010).
Singh, S., Carpenter, A.E. & Genovesio, A. Increasing the content of high-content screening: an overview. J. Biomol. Screen. 19, 640–650 (2014).
Perlman, Z.E. et al. Multidimensional drug profiling by automated microscopy. Science 306, 1194–1198 (2004).
Danuser, G. Computer vision in cell biology. Cell 147, 973–978 (2011).
Altschuler, S.J. & Wu, L.F. Cellular heterogeneity: do differences make a difference? Cell 141, 559–563 (2010).
Snijder, B. & Pelkmans, L. Origins of regulated cell-to-cell variability. Nat. Rev. Mol. Cell Biol. 12, 119–125 (2011).
Eliceiri, K.W. et al. Biological imaging software tools. Nat. Methods 9, 697–710 (2012).
Paull, K.D. et al. Display and analysis of patterns of differential activity of drugs against human tumor cell lines: development of mean graph and COMPARE algorithm. J. Natl. Cancer Inst. 81, 1088–1092 (1989).
Lamb, J. et al. The connectivity map: using gene-expression signatures to connect small molecules, genes, and disease. Science 313, 1929–1935 (2006).
Adams, C.L. et al. Compound classification using image-based cellular phenotypes. Methods Enzymol. 414, 440–468 (2006).
Loo, L.-H., Wu, L.F. & Altschuler, S.J. Image-based multivariate profiling of drug responses from single cells. Nat. Methods 4, 445–453 (2007).
Young, D.W. et al. Integrating high-content screening and ligand-target prediction to identify mechanism of action. Nat. Chem. Biol. 4, 59–68 (2008).
Ljosa, V. et al. Comparison of methods for image-based profiling of cellular morphological responses to small-molecule treatment. J. Biomol. Screen. 18, 1321–1329 (2013).
Reisen, F. et al. Linking phenotypes and modes of action through high-content screen fingerprints. Assay Drug Dev. Technol. 13, 415–427 (2015).
Futamura, Y. et al. Morphobase, an encyclopedic cell morphology database, and its use for drug target identification. Chem. Biol. 19, 1620–1630 (2012).
Sundaramurthy, V. et al. Integration of chemical and RNAi multiparametric profiles identifies triggers of intracellular mycobacterial killing. Cell Host Microbe 13, 129–142 (2013).
Castoreno, A.B. et al. Small molecules discovered in a pathway screen target the Rho pathway in cytokinesis. Nat. Chem. Biol. 6, 457–463 (2010).
Loo, L.-H. et al. An approach for extensibly profiling the molecular states of cellular subpopulations. Nat. Methods 6, 759–765 (2009).
Fuchs, F. et al. Clustering phenotype populations by genome-wide RNAi and multiparametric imaging. Mol. Syst. Biol. 6, 370 (2010).
Collinet, C. et al. Systems survey of endocytosis by multiparametric image analysis. Nature 464, 243–249 (2010).
Laufer, C., Fischer, B., Billmann, M., Huber, W. & Boutros, M. Mapping genetic interactions in human cancer cells with RNAi and multiparametric phenotyping. Nat. Methods 10, 427–431 (2013).
Liberali, P., Snijder, B. & Pelkmans, L. A hierarchical map of regulatory genetic interactions in membrane trafficking. Cell 157, 1473–1487 (2014).
Fischer, B. et al. A map of directional genetic interactions in a metazoan cell. Elife 4, e05464 (2015).
Yin, Z. et al. A screen for morphological complexity identifies regulators of switch-like transitions between discrete cell shapes. Nat. Cell Biol. 15, 860–871 (2013).
Gustafsdottir, S.M. et al. Multiplex cytological profiling assay to measure diverse cellular states. PLoS One 8, e80999 (2013).
Wawer, M.J. et al. Toward performance-diverse small-molecule libraries for cell-based phenotypic screening using multiplexed high-dimensional profiling. Proc. Natl. Acad. Sci. USA 111, 10911–10916 (2014).
Singh, S. et al. Morphological profiles of RNAi-induced gene knockdown are highly reproducible but dominated by seed effects. PLoS One 10, e0131370 (2015).
Gibson, C.C. et al. Strategy for identifying repurposed drugs for the treatment of cerebral cavernous malformation. Circulation 131, 289–299 (2015).
MacRae, C.A. A new phenotypic lexicon for accelerated translation: rise of the machines. Circulation 131, 234–236 (2015).
Petrone, P.M. et al. Biodiversity of small molecules--a new perspective in screening set selection. Drug Discov. Today 18, 674–680 (2013).
Peck, D. et al. A method for high-throughput gene expression signature analysis. Genome Biol. 7, R61 (2006).
Rajaram, S., Pavie, B., Wu, L.F. & Altschuler, S.J. PhenoRipper: software for rapidly profiling microscopy images. Nat. Methods 9, 635–637 (2012).
Hartwell, K.A. et al. Niche-based screening identifies small-molecule inhibitors of leukemia stem cells. Nat. Chem. Biol. 9, 840–848 (2013).
Uhlmann, V., Singh, S. & Carpenter, A.E. CP-CHARM: segmentation-free image classification made accessible. BMC Bioinformatics 17, 51 (2016).
Bray, M.-A. & Carpenter, A. in Assay Guidance Manual (eds. Sittampalam, G.S. et al.) (Eli Lilly & Company and the National Center for Advancing Translational Sciences, 2013).
Iversen, P.W. et al. in Assay Guidance Manual (eds. Sittampalam, G.S. et al.) (Eli Lilly & Company and the National Center for Advancing Translational Sciences, 2012).
Singh, S., Bray, M.-A., Jones, T.R. & Carpenter, A.E. Pipeline for illumination correction of images for high-throughput microscopy. J. Microsc. 256, 231–236 (2014).
Bray, M.-A., Fraser, A.N., Hasaka, T.P. & Carpenter, A.E. Workflow and metrics for image quality control in large-scale high-content screens. J. Biomol. Screen. 17, 266–274 (2012).
Clarke, R. et al. The properties of high-dimensional data spaces: implications for exploring gene and protein expression data. Nat. Rev. Cancer 8, 37–49 (2008).
Feng, Y., Mitchison, T.J., Bender, A., Young, D.W. & Tallarico, J.A. Multi-parameter phenotypic profiling: using cellular effects to characterize small-molecule compounds. Nat. Rev. Drug Discov. 8, 567–578 (2009).
Janzen, W.P. & Popa-Burke, I.G. Advances in improving the quality and flexibility of compound management. J. Biomol. Screen. 14, 444–451 (2009).
Lundholt, B.K., Scudder, K.M. & Pagliaro, L. A simple technique for reducing edge effect in cell-based assays. J. Biomol. Screen. 8, 566–570 (2003).
Ljosa, V., Sokolnicki, K.L. & Carpenter, A.E. Annotated high-throughput microscopy image sets for validation. Nat. Methods 9, 637 (2012).
Guyon, I. & Elisseeff, A. An introduction to variable and feature selection. J. Mach. Learn. Res. 3, 1157–1182 (2003).
Carpenter, A.E. et al. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 7, R100 (2006).
Acknowledgements
Research reported in this publication was supported in part by NIH R44 TR001197 (C.C.G.), NSF RIG DBI 1119830 (M.-A.B.), NIH R01 GM089652 (A.E.C.), and NSF CAREER DBI 1148823 (A.E.C.). The RNAi Cell Painting knockdown experiment used in this paper was previously published31 and was supported in part by the Slim Initiative for Genomic Medicine, a project funded by the Carlos Slim Foundation in Mexico. The authors thank the original developers of earlier versions of the protocol, who are the authors of the original paper describing the assay29; these authors are S.M. Gustafsdottir, V. Ljosa, K.L. Sokolnicki, J.A. Wilson, D. Walpita, M.M. Kemp, K. Petri Seiler, H.A. Carrel, T.R. Golub, S.L. Schreiber, P.A. Clemons, A.E. Carpenter, and A.F. Shamji. For enabling this work, Recursion Pharmaceuticals thanks the University of Utah Core Facilities (J. Phillips), specifically the Drug Discovery Core (B. Luo) and the Fluorescent Imaging Core (C. Rodesch). We thank members of the Broad Institute's Center for the Development of Therapeutics, especially T.P. Hasaka, for technical assistance. We also thank A. Kozol for proofreading the Equipment and Reagents sections and for testing the image analysis workflow, and A. Berger and X. Wu for offering helpful comments and suggestions during manuscript preparation.
Author information
Authors and Affiliations
Contributions
All authors contributed to writing this protocol. C.T.D., H.H., and S.M.G. contributed to describing the benchwork aspects of the protocol. C.H., M.K.-A., M.-A.B., and C.T.D. contributed to updates to the experimental design. M.-A.B. and S.S. contributed most heavily to describing the computational aspects of the protocol. A.E.C., C.C.G., B.B., and S.S. contributed most heavily to describing the rationale and the experimental design.
Corresponding authors
Ethics declarations
Competing interests
Recursion Pharmaceuticals is a biotechnology company in which C.C.G., B.B., C.T.D., H.H., and A.E.C. have real or optional ownership interest.
Supplementary information
Supplementary Method
Steps for producing per-well morphological profiles from single-cell measurements (PDF 163 kb)
Supplementary Note
Imaging considerations for wide-field versus confocal microscopes (PDF 308 kb)
Rights and permissions
About this article
Cite this article
Bray, MA., Singh, S., Han, H. et al. Cell Painting, a high-content image-based assay for morphological profiling using multiplexed fluorescent dyes. Nat Protoc 11, 1757–1774 (2016). https://doi.org/10.1038/nprot.2016.105
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2016.105
This article is cited by
-
High-content analysis identified synergistic drug interactions between INK128, an mTOR inhibitor, and HDAC inhibitors in a non-small cell lung cancer cell line
BMC Cancer (2024)
-
Pooled multicolour tagging for visualizing subcellular protein dynamics
Nature Cell Biology (2024)
-
High-dimensional phenotyping to define the genetic basis of cellular morphology
Nature Communications (2024)
-
Merging bioactivity predictions from cell morphology and chemical fingerprint models using similarity to training data
Journal of Cheminformatics (2023)
-
A semiconductor 96-microplate platform for electrical-imaging based high-throughput phenotypic screening
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.