Abstract
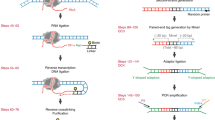
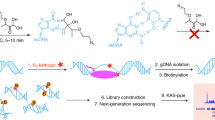
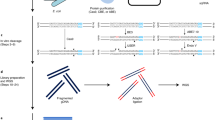
We provide a protocol for precision nuclear run-on sequencing (PRO-seq) and its variant, PRO-cap, which map the location of active RNA polymerases (PRO-seq) or transcription start sites (TSSs) (PRO-cap) genome-wide at high resolution. The density of RNA polymerases at a particular genomic locus directly reflects the level of nascent transcription at that region. Nuclei are isolated from cells and, under nuclear run-on conditions, transcriptionally engaged RNA polymerases incorporate one or, at most, a few biotin-labeled nucleotide triphosphates (biotin-NTPs) into the 3′ end of nascent RNA. The biotin-labeled nascent RNA is used to prepare sequencing libraries, which are sequenced from the 3′ end to provide high-resolution positional information for the RNA polymerases. PRO-seq provides much higher sensitivity than ChIP-seq, and it generates a much larger fraction of usable sequence reads than ChIP-seq or NET-seq (native elongating transcript sequencing). Similarly to NET-seq, PRO-seq maps the RNA polymerase at up to base-pair resolution with strand specificity, but unlike NET-seq it does not require immunoprecipitation. With the protocol provided here, PRO-seq (or PRO-cap) libraries for high-throughput sequencing can be generated in 4–5 working days. The method has been applied to human, mouse, Drosophila melanogaster and Caenorhabditis elegans cells and, with slight modifications, to yeast.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Fuda, N.J., Ardehali, M.B. & Lis, J.T. Defining mechanisms that regulate RNA polymerase II transcription in vivo. Nature 461, 186–192 (2009).
Adelman, K. & Lis, J.T. Promoter-proximal pausing of RNA polymerase II: emerging roles in metazoans. Nat. Rev. Genet. 13, 720–731 (2012).
Core, L.J. et al. Analysis of nascent RNA identifies a unified architecture of initiation regions at mammalian promoters and enhancers. Nat. Genet. 46, 1311–1320 (2014).
Heinz, S., Romanoski, C.E., Benner, C. & Glass, C.K. The selection and function of cell type-specific enhancers. Nat. Rev. Mol. Cell Biol. 16, 144–154 (2015).
Vahedi, G. et al. Super-enhancers delineate disease-associated regulatory nodes in T cells. Nature 520, 558–562 (2015).
Churchman, L.S. & Weissman, J.S. Nascent transcript sequencing visualizes transcription at nucleotide resolution. Nature 469, 368–373 (2011).
Larson, M.H. et al. A pause sequence enriched at translation start sites drives transcription dynamics in vivo. Science 344, 1042–1047 (2014).
Nojima, T. et al. Mammalian NET-Seq reveals genome-wide nascent transcription coupled to RNA processing. Cell 161, 526–540 (2015).
Weber, C.M., Ramachandran, S. & Henikoff, S. Nucleosomes are context-specific, H2A.Z-modulated barriers to RNA polymerase. Mol. Cell. 53, 819–830 (2014).
Core, L.J., Waterfall, J.J. & Lis, J.T. Nascent RNA sequencing reveals widespread pausing and divergent initiation at human promoters. Science 322, 1845–1848 (2008).
Kwak, H., Fuda, N.J., Core, L.J. & Lis, J.T. Precise maps of RNA polymerase reveal how promoters direct initiation and pausing. Science 339, 950–953 (2013).
Jonkers, I., Kwak, H. & Lis, J.T. Genome-wide dynamics of Pol II elongation and its interplay with promoter proximal pausing, chromatin, and exons. Elife 3, e02407 (2014).
Kwak, H. & Lis, J.T. Control of transcriptional elongation. Annu. Rev. Genet. 47, 483–508 (2013).
Hah, N. et al. A rapid, extensive, and transient transcriptional response to estrogen signaling in breast cancer cells. Cell 145, 622–634 (2011).
Min, I.M. et al. Regulating RNA polymerase pausing and transcription elongation in embryonic stem cells. Genes Dev. 25, 742–754 (2011).
Larschan, E. et al. X chromosome dosage compensation via enhanced transcriptional elongation in Drosophila. Nature 471, 115–118 (2011).
Cock, P.J.A., Fields, C.J., Goto, N., Heuer, M.L. & Rice, P.M. The Sanger FASTQ file format for sequences with quality scores, and the Solexa/Illumina FASTQ variants. Nucl. Acids Res. 38, 1767–1771 (2010).
Core, L.J. et al. Defining the status of RNA polymerase at promoters. Cell Rep. 2, 1025–1035 (2012).
Seila, A.C. et al. Divergent transcription from active promoters. Science 322, 1849–1851 (2008).
Carninci, P. et al. High-efficiency full-length cDNA cloning by biotinylated CAP trapper. Genomics 37, 327–336 (1996).
Andersson, R. et al. An atlas of active enhancers across human cell types and tissues. Nature 507, 455–461 (2014).
Forrest, A.R.R. et al. A promoter-level mammalian expression atlas. Nature 507, 462–470 (2014).
Wang, D. et al. Reprogramming transcription by distinct classes of enhancers functionally defined by eRNA. Nature 474, 390–394 (2011).
Hah, N., Murakami, S., Nagari, A., Danko, C.G. & Kraus, W.L. Enhancer transcripts mark active estrogen receptor binding sites. Genome Res. 23, 1210–1223 (2013).
Fejes-Toth, K. et al. Post-transcriptional processing generates a diversity of 5-modified long and short RNAs. Nature 457, 1028–1032 (2009).
Rhee, H.S. & Pugh, B.F. Genome-wide structure and organization of eukaryotic pre-initiation complexes. Nature 483, 295–301 (2012).
Li, J. et al. Kinetic competition between elongation rate and binding of NELF controls promoter-proximal pausing. Mol. Cell 50, 711–722 (2013).
Mayer, A. et al. Native elongating transcript sequencing reveals human transcriptional activity at nucleotide resolution. Cell 161, 541–554 (2015).
Mahat, D.B., Salamanca, H.H., Duarte, F.M., Danko, C.G. & Lis, J.T. Mammalian heat shock response and mechanisms underlying its genome-wide transcriptional regulation. Mol. Cell 62, 63–78 (2016).
García-Martínez, J., Aranda, A. & Pérez-Ortín, J.E. Genomic run-on evaluates transcription rates for all yeast genes and identifies gene regulatory mechanisms. Mol. Cell 15, 303–313 (2004).
Collart, M.A. & Oliviero, S. Preparation of yeast RNA. Curr. Protoc. Mol. Biol. Chapter 13, Unit13.12 (2001).
Job, D. et al. Complex RNA chain elongation kinetics by wheat germ RNA polymerase II. Nucleic Acids Res. 12, 3303–3319 (1984).
Fu, G.K. et al. Molecular indexing enables quantitative targeted RNA sequencing and reveals poor efficiencies in standard library preparations. Proc. Natl. Acad. Sci. USA 111, 1891–1896 (2014).
Marcel, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17, 10–12 (2011).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Langmead, B., Trapnell, C., Pop, M. & Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25 (2009).
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Quinlan, A.R. & Hall, I.M. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842 (2010).
Acknowledgements
We thank all the present and former members of the Lis lab who provided advice and support for this work. We especially thank N. Fuda, a former member of our lab, for help at many steps of this protocol. Research reported in this publication was supported by National Institutes of Health (NIH) grant R01GM25232 to J.T.L. and European Research Council Advanced Grant ERCadv-671274 to I.H.J. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author information
Authors and Affiliations
Contributions
H.K., L.J.C. and J.T.L. conceived the method and designed the experiments. H.K. and D.B.M. carried out the experiments that generated the data. G.T.B., I.H.J., R.K.P., C.T.W., K.M. and L.J.C. carried out experiments that optimized the protocol. C.G.D. contributed in generating the pipelines for the computational analysis. H.K., D.B.M. and J.T.L. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Tables 1–9
(PDF 162 kb)
Rights and permissions
About this article
Cite this article
Mahat, D., Kwak, H., Booth, G. et al. Base-pair-resolution genome-wide mapping of active RNA polymerases using precision nuclear run-on (PRO-seq). Nat Protoc 11, 1455–1476 (2016). https://doi.org/10.1038/nprot.2016.086
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2016.086
This article is cited by
-
Internal and external normalization of nascent RNA sequencing run-on experiments
BMC Bioinformatics (2024)
-
Precision run-on sequencing (PRO-seq) for microbiome transcriptomics
Nature Microbiology (2024)
-
RNA polymerase II pausing is essential during spermatogenesis for appropriate gene expression and completion of meiosis
Nature Communications (2024)
-
Nascent transcription upon interferon-α2 stimulation on human and rhesus macaque lymphoblastoid cell lines
BMC Research Notes (2023)
-
Cis-regulatory atlas of primary human CD4+ T cells
BMC Genomics (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.