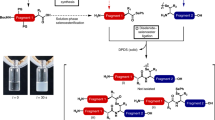
Abstract
Total chemical synthesis of proteins allows researchers to custom design proteins without the complex molecular biology that is required to insert non-natural amino acids or the biocontamination that arises from methods relying on overexpression in cells. We describe a detailed procedure for the chemical synthesis of proteins with the α-ketoacid–hydroxylamine (KAHA ligation), using (S)-5-oxaproline (Opr) as a key building block. This protocol comprises two main parts: (i) the synthesis of peptide fragments by standard fluorenylmethoxycarbonyl (Fmoc) chemistry and (ii) the KAHA ligation between fragments containing Opr and a C-terminal peptide α-ketoacid. This procedure provides an alternative to native chemical ligation (NCL) that could be valuable for the synthesis of proteins, particularly targets that do not contain cysteine residues. The ligation conditions—acidic DMSO/H2O or N-methyl-2-pyrrolidinone (NMP)/H2O—are ideally suited for solubilizing peptide segments, including many hydrophobic examples. The utility and efficiency of the protocol is demonstrated by the total chemical synthesis of the mature betatrophin (also called ANGPTL8), a 177-residue protein that contains no cysteine residues. With this protocol, the total synthesis of the betatrophin protein has been achieved in around 35 working days on a multimilligram scale.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kent, S.B. Total chemical synthesis of proteins. Chem. Soc. Rev. 38, 338–351 (2009).
Dawson, P.E., Muir, T.W., Clark-Lewis, I. & Kent, S.B. Synthesis of proteins by native chemical ligation. Science 266, 776–779 (1994).
Yan, L.Z. & Dawson, P.E. Synthesis of peptides and proteins without cysteine residues by native chemical ligation combined with desulfurization. J. Am. Chem. Soc. 123, 526–533 (2001).
Hojo, H., Onuma, Y., Akimoto, Y., Nakahara, Y. & Nakahara, Y. N-Alkyl cysteine-assisted thioesterification of peptides. Tetrahedron Lett. 48, 25–28 (2007).
Wan, Q. & Danishefsky, S.J. Free-radical-based, specific desulfurization of cysteine: a powerful advance in the synthesis of polypeptides and glycopolypeptides. Angew. Chem. Int. Ed. 46, 9248–9252 (2007).
Blanco-Canosa, J.B. & Dawson, P.E. An efficient Fmoc-SPPS approach for the generation of thioester peptide precursors for use in native chemical ligation. Angew. Chem. Int. Ed. 47, 6851–6855 (2008).
Tsuda, S., Shigenaga, A., Bando, K. & Otaka, A. N→S acyl-transfer-mediated synthesis of peptide thioesters using anilide derivatives. Org. Lett. 11, 823–826 (2009).
Erlich, L.A., Kumar, K.S., Haj-Yahya, M., Dawson, P.E. & Brik, A. N-methylcysteine-mediated total chemical synthesis of ubiquitin thioester. Org. Biomol. Chem. 8, 2392–2396 (2010).
Zheng, J.-S., Tang, S., Qi, Y.-K., Wang, Z.-P. & Liu, L. Chemical synthesis of proteins using peptide hydrazides as thioester surrogates. Nat. Protoc. 8, 2483–2495 (2013).
Boll, E. et al. One-pot chemical synthesis of small ubiquitin-like modifier protein–peptide conjugates using bis(2-sulfanylethyl)amido peptide latent thioester surrogates. Nat. Protoc. 10, 269–292 (2015).
Valverde, I.E., Lecaille, F., Lalmanach, G., Aucagne, V. & Delmas, A.F. Synthesis of a biologically active triazole-containing analogue of cystatin A through successive peptidomimetic alkyne-azide ligations. Angew. Chem. Int. Ed. 51, 718–722 (2012).
Zhang, Y., Xu, C., Lam, H.Y., Lee, C.L. & Li, X. Protein chemical synthesis by serine and threonine ligation. Proc. Natl. Acad. Sci. USA 110, 6657–6662 (2013).
Harmand, T.J., Murar, C.E. & Bode, J.W. New chemistries for chemoselective peptide ligations and the total synthesis of proteins. Curr. Opin. Chem. Biol. 22, 115–121 (2014).
Pattabiraman, V.R., Ogunkoya, A.O. & Bode, J.W. Chemical protein synthesis by chemoselective α-ketoacid–hydroxylamine (KAHA) ligations with 5-oxaproline. Angew. Chem. Int. Ed. 51, 5114–5118 (2012).
Ogunkoya, A.O., Pattabiraman, V.R. & Bode, J.W. Sequential α-ketoacid-hydroxylamine (KAHA) ligations: synthesis of C-terminal variants of the modifier protein UFM1. Angew. Chem. Int. Ed. 51, 9693–9697 (2012).
Wucherpfennig, T.G., Rohrbacher, F., Pattabiraman, V.R. & Bode, J.W. Formation and rearrangement of homoserine depsipeptides and depsiproteins in the alpha-ketoacid-hydroxylamine ligation with 5-oxaproline. Angew. Chem. Int. Ed. 53, 12244–12247 (2014).
Wucherpfennig, T.G., Pattabiraman, V.R., Limberg, F.R., Ruiz-Rodriguez, J. & Bode, J.W. Traceless preparation of C-terminal alpha-ketoacids for chemical protein synthesis by alpha-ketoacid-hydroxylamine ligation: synthesis of SUMO2/3. Angew. Chem. Int. Ed. 53, 12248–12252 (2014).
Pusterla, I. & Bode, J.W. An oxazetidine amino acid for chemical protein synthesis by rapid, serine-forming ligations. Nat. Chem. 7, 668–672 (2015).
He, C., Kulkarni, S.S., Thuaud, F. & Bode, J.W. Chemical synthesis of the 20 kDa heme protein nitrophorin 4 by alpha-ketoacid-hydroxylamine (KAHA) ligation. Angew. Chem. Int. Ed. 54, 12996–13001 (2015).
Harmand, T.J., Kulkarni, S.S. & Bode, J.W. Optimized synthesis of a cyanosulfurylide linker for Fmoc-SPPS of C-terminal peptide α-ketoacids. Tetrahedron Lett. 56, 3477–3480 (2015).
Murar, C.E., Thuaud, F. & Bode, J.W. KAHA ligations that form aspartyl aldehyde residues as synthetic handles for protein modification and purification. J. Am. Chem. Soc. 136, 18140–18148 (2014).
Yi, P., Park, J.S. & Melton, D.A. Betatrophin: a hormone that controls pancreatic beta cell proliferation. Cell 153, 747–758 (2013).
Wang, Y. et al. Mice lacking ANGPTL8 (betatrophin) manifest disrupted triglyceride metabolism without impaired glucose homeostasis. Proc. Natl. Acad. Sci. USA 110, 16109–16114 (2013).
Gusarova, V. et al. ANGPTL8/betatrophin does not control pancreatic beta cell expansion. Cell 159, 691–696 (2014).
Gómez-Ambrosi, J. et al. Circulating betatrophin concentrations are decreased in human obesity and type 2 diabetes. J. Clin. Endocrinol. Metab. 99, E2004–E2009 (2014).
Yi, P., Park, J.S. & Melton, D.A. Perspectives on the activities of ANGPTL8/betatrophin. Cell 159, 467–468 (2014).
Weinstock, M.T., Jacobsen, M.T. & Kay, M.S. Synthesis and folding of a mirror-image enzyme reveals ambidextrous chaperone activity. Proc. Natl. Acad. Sci. USA 111, 11679–11684 (2014).
Seenaiah, M., Jbara, M., Mali, S.M. & Brik, A. Convergent versus sequential protein synthesis: the case of ubiquitinated and glycosylated H2B. Angew. Chem. Int. Ed. 54, 12374–12378 (2015).
Yung, A., Papworth-Smith, J. & Wilkinson, S.M. Occupational contact urticaria from the solid-phase peptide synthesis coupling agents HATU and HBTU. Contact Dermatitis 49, 108–109 (2003).
Bang, D. & Kent, S.B. A one-pot total synthesis of crambin. Angew. Chem. Int. Ed. 43, 2534–2538 (2004).
Tang, S. et al. An efficient one-pot four-segment condensation method for protein chemical synthesis. Angew. Chem. Int. Ed. 54, 5713–5717 (2015).
Veber, D., Milkowski, J., Varga, S., Denkewalter, R. & Hirschmann, R. Acetamidomethyl. A novel thiol protecting group for cysteine. J. Am. Chem. Soc. 94, 5456–5461 (1972).
Gude, M., Ryf, J. & White, P. An accurate method for the quantitation of Fmoc-derivatized solid phase supports. Lett. Pept. Sci. 9, 203–206 (2002).
Acknowledgements
This work was supported by the ETH Zürich and the Swiss National Science Foundation (200020_150073). We thank the LOC MS Service for analyses and T. Hayashi for his help with folding and CD spectrum measurement.
Author information
Authors and Affiliations
Contributions
T.J.H. and C.E.M. performed the experiments, compound characterization and data analysis. All authors contributed to experimental design, discussions and writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 SDS-PAGE and CD spectra of purified betatrophin 9
(a) SDS-PAGE/Coomassie staining analysis of purified synthetic betatrophin 9. (b) circular dichroism spectra of refolded synthetic betatrophin 9. ~20μM of betatrophin 9 in PBS buffer pH 7.2 was analyzed in a 1 mm quartz cell.
Supplementary information
Supplementary Text and Figures
Supplementary Figure 1, Supplementary Data 1–9 (PDF 2286 kb)
Rights and permissions
About this article
Cite this article
Harmand, T., Murar, C. & Bode, J. Protein chemical synthesis by α-ketoacid–hydroxylamine ligation. Nat Protoc 11, 1130–1147 (2016). https://doi.org/10.1038/nprot.2016.052
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2016.052
This article is cited by
-
A biocatalytic platform for asymmetric alkylation of α-keto acids by mining and engineering of methyltransferases
Nature Communications (2023)
-
Diselenide–selenoester ligation for chemical protein synthesis
Nature Protocols (2019)
-
Thirteen decades of peptide synthesis: key developments in solid phase peptide synthesis and amide bond formation utilized in peptide ligation
Amino Acids (2018)
-
Total chemical synthesis of histones and their analogs, assisted by native chemical ligation and palladium complexes
Nature Protocols (2017)
-
Recent advances in the preparation of Fmoc-SPPS-based peptide thioester and its surrogates for NCL-type reactions
Science China Chemistry (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.