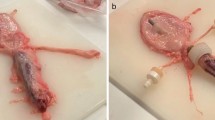
Abstract
Venous access catheters used in clinics are prone to biofilm contamination, contributing to chronic and nosocomial infections. Although several animal models for studying device-associated biofilms were previously described, only a few detailed protocols are currently available. Here we provide a protocol using totally implantable venous access ports (TIVAPs) implanted in rats. This model recapitulates all phenomena observed in the clinic, and it allows bacterial biofilm development and physiology to be studied. After TIVAP implantation and inoculation with luminescent pathogens, in vivo biofilm formation can be monitored in situ, and biofilm biomass can be recovered from contaminated TIVAP and organs. We used this protocol to study host responses to biofilm infection, to evaluate preventive and curative antibiofilm strategies and to study fundamental biofilm properties. For this procedure, one should expect ∼3 h of hands-on time, including the implantation in one rat followed by in situ luminescence monitoring and bacterial load estimation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Percival, S.L., Suleman, L., Vuotto, C. & Donelli, G. Healthcare-associated infections, medical devices and biofilms: risk, tolerance and control. J. Med. Microbiol. 64, 323–334 (2015).
Hoiby, N. et al. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin. Microbiol. Infect. 21 (suppl. 1): S1–S25 (2015).
Lebeaux, D. et al. Management of infections related to totally implantable venous-access ports: challenges and perspectives. Lancet Infect. Dis. 14, 146–159 (2014).
Mermel, L.A. et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 49, 1–45 (2009).
Bouza, E., Perez-Molina, J.A. & Munoz, P. Report of ESGNI-001 and ESGN2-002 studies. Bloodstream infections in Europe. Clin. Microbiol. Infect. 5, 2S1–2S12 (1999).
Ceri, H. et al. The Calgary Biofilm Device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 37, 1771–1776 (1999).
Coenye, T. & Nelis, H.J. In vitro and in vivo model systems to study microbial biofilm formation. J. Microbiol. Methods 83, 89–105 (2010).
Donlan, R.M. et al. Model system for growing and quantifying Streptococcus pneumoniae biofilms in situ and in real time. Appl. Environ. Microbiol. 70, 4980–4988 (2004).
Goeres, D.M. et al. Statistical assessment of a laboratory method for growing biofilms. Microbiology 151, 757–762 (2005).
Lebeaux, D., Chauhan, A., Rendueles, O. & Beloin, C. From in vitro to in vivo models of bacterial biofilm-related infections. Pathogens 2, 288–356 (2013).
Parra-Ruiz, J., Vidaillac, C., Rose, W.E. & Rybak, M.J. Activities of high-dose daptomycin, vancomycin, and moxifloxacin alone or in combination with clarithromycin or rifampin in a novel in vitro model of Staphylococcus aureus biofilm. Antimicrob. Agents Chemother. 54, 4329–4334 (2010).
Van Wijngaerden, E. et al. Foreign body infection: a new rat model for prophylaxis and treatment. J. Antimicrob. Chemother. 44, 669–674 (1999).
Kadurugamuwa, J.L. et al. Direct continuous method for monitoring biofilm infection in a mouse model. Infect. Immun. 71, 882–890 (2003).
Kucharikova, S., Vande Velde, G., Himmelreich, U. & Van Dijck, P. Candida albicans biofilm development on medically-relevant foreign bodies in a mouse subcutaneous model followed by bioluminescence imaging. J. Vis. Exp. doi:10.3791/52239 (2015).
Vande Velde, G., Kucharikova, S., Schrevens, S., Himmelreich, U. & Van Dijck, P. Towards non-invasive monitoring of pathogen-host interactions during Candida albicans biofilm formation using in vivo bioluminescence. Cell. Microbiol. 16, 115–130 (2014).
Vuong, C., Kocianova, S., Yu, J., Kadurugamuwa, J.L. & Otto, M. Development of real-time in vivo imaging of device-related Staphylococcus epidermidis infection in mice and influence of animal immune status on susceptibility to infection. J. Infect. Dis. 198, 258–261 (2008).
Wang, R. et al. Staphylococcus epidermidis surfactant peptides promote biofilm maturation and dissemination of biofilm-associated infection in mice. J. Clin. Invest. 121, 238–248 (2011).
Cirioni, O. et al. RNAIII-inhibiting peptide significantly reduces bacterial load and enhances the effect of antibiotics in the treatment of central venous catheter-associated Staphylococcus aureus infections. J. Infect. Dis. 193, 180–186 (2006).
Li, H. et al. Conversion of Staphylococcus epidermidis strains from commensal to invasive by expression of the ica locus encoding production of biofilm exopolysaccharide. Infect. Immun. 73, 3188–3191 (2005).
Rupp, M.E., Ulphani, J.S., Fey, P.D. & Mack, D. Characterization of Staphylococcus epidermidis polysaccharide intercellular adhesin/hemagglutinin in the pathogenesis of intravascular catheter-associated infection in a rat model. Infect. Immun. 67, 2656–2659 (1999).
Ulphani, J.S. & Rupp, M.E. Model of Staphylococcus aureus central venous catheter-associated infection in rats. Lab. Anim. Sci. 49, 283–287 (1999).
Lorenz, U. et al. The alternative sigma factor sigma B of Staphylococcus aureus modulates virulence in experimental central venous catheter-related infections. Microbes Infect. 10, 217–223 (2008).
Kokai-Kun, J.F., Chanturiya, T. & Mond, J.J. Lysostaphin eradicates established Staphylococcus aureus biofilms in jugular vein catheterized mice. J. Antimicrob. Chemother. 64, 94–100 (2009).
Fernandez-Hidalgo, N. et al. Evaluation of linezolid, vancomycin, gentamicin and ciprofloxacin in a rabbit model of antibiotic-lock technique for Staphylococcus aureus catheter-related infection. J. Antimicrob. Chemother. 65, 525–530 (2010).
Andes, D. et al. Development and characterization of an in vivo central venous catheter Candida albicans biofilm model. Infect. Immun. 72, 6023–6031 (2004).
Lazzell, A.L. et al. Treatment and prevention of Candida albicans biofilms with caspofungin in a novel central venous catheter murine model of candidiasis. J. Antimicrob. Chemother. 64, 567–570 (2009).
Li, F. et al. Eap1p, an adhesin that mediates Candida albicans biofilm formation in vitro and in vivo. Eukaryot. Cell 6, 931–939 (2007).
Schinabeck, M.K. et al. Rabbit model of Candida albicans biofilm infection: liposomal amphotericin B antifungal lock therapy. Antimicrob. Agents Chemother. 48, 1727–1732 (2004).
Chauhan, A. et al. Preventing biofilm formation and associated occlusion by biomimetic glycocalyxlike polymer in central venous catheters. J. Infect. Dis. 210, 1347–1356 (2014).
Chauhan, A. et al. A rat model of central venous catheter to study establishment of long-term bacterial biofilm and related acute and chronic infections. PLoS ONE 7, e37281 (2012).
Chauhan, A., Lebeaux, D., Ghigo, J.M. & Beloin, C. Full and broad-spectrum in vivo eradication of catheter-associated biofilms using gentamicin-EDTA antibiotic lock therapy. Antimicrob. Agents Chemother. 56, 6310–6318 (2012).
Chalabaev, S. et al. Biofilms formed by Gram-negative bacteria undergo increased lipid a palmitoylation, enhancing in vivo survival. Mbio 5, e01116 (2014).
Lebeaux, D. et al. pH-mediated potentiation of aminoglycosides kills bacterial persisters and eradicates in vivo biofilms. J. Infect. Dis. 210, 1357–1366 (2014).
Funao, H. et al. Establishment of a real-time, quantitative, and reproducible mouse model of Staphylococcus osteomyelitis using bioluminescence imaging. Infect. Immun. 80, 733–741 (2012).
Daghighi, S. et al. Persistence of a bioluminescent Staphylococcus aureus strain on and around degradable and non-degradable surgical meshes in a murine model. Acta Biomater. 8, 3991–3996 (2012).
Li, D. et al. Quantitative mouse model of implant-associated osteomyelitis and the kinetics of microbial growth, osteolysis, and humoral immunity. J. Orthop. Res. 26, 96–105 (2008).
Hertlein, T. et al. Bioluminescence and 19F magnetic resonance imaging visualize the efficacy of lysostaphin alone and in combination with oxacillin against Staphylococcus aureus in murine thigh and catheter-associated infection models. Antimicrob. Agents Chemother. 58, 1630–1638 (2014).
Fatkenheuer, G. et al. Central venous catheter (CVC)-related infections in neutropenic patients—guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO). Ann. Hematol. 82 (suppl. 2), S149–S157 (2003).
Messing, B., Peitra-Cohen, S., Debure, A., Beliah, M. & Bernier, J.J. Antibiotic-lock technique: a new approach to optimal therapy for catheter-related sepsis in home-parenteral nutrition patients. JPEN J. Parenter. Enteral Nutr. 12, 185–189 (1988).
Zeng, G., Ogaki, R. & Meyer, R.L. Non-proteinaceous bacterial adhesins challenge the antifouling properties of polymer brush coatings. Acta Biomater. 24, 64–73 (2015).
Mussard, W., Kebir, N., Kriegel, I., Esteve, M. & Semetey, V. Facile and efficient control of bioadhesion on poly(dimethylsiloxane) by using a biomimetic approach. Angew. Chem. Int. Ed. Engl. 50, 10871–10874 (2011).
van Rooden, C.J. et al. Infectious complications of central venous catheters increase the risk of catheter-related thrombosis in hematology patients: a prospective study. J. Clin. Oncol. 23, 2655–2660 (2005).
Kilkenny, C., Browne, W.J., Cuthill, I.C., Emerson, M. & Altman, D.G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 8, e1000412 (2010).
Ramphal, R. et al. Control of Pseudomonas aeruginosa in the lung requires the recognition of either lipopolysaccharide or flagellin. J. Immunol. 181, 586–592 (2008).
Bernier, C., Gounon, P. & Le Bouguenec, C. Identification of an aggregative adhesion fimbria (AAF) type III-encoding operon in enteroaggregative Escherichia coli as a sensitive probe for detecting the AAF-encoding operon family. Infect. Immun. 70, 4302–4311 (2002).
Foucault, M.L., Thomas, L., Goussard, S., Branchini, B.R. & Grillot-Courvalin, C. In vivo bioluminescence imaging for the study of intestinal colonization by Escherichia coli in mice. Appl. Environ. Microbiol. 76, 264–274 (2010).
Rutala, W.A., Weber, D.J. & Healthcare Infection Control Practices Advisory Committee (HICPAC) Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008. Centers for Disease Control and Prevention (2010).
Van Herck, H. et al. Blood sampling from the retro-orbital plexus, the saphenous vein and the tail vein in rats: comparative effects on selected behavioural and blood variables. Lab. Anim. 35, 131–139 (2001).
van Herck, H. et al. Orbital sinus blood sampling in rats as performed by different animal technicians: the influence of technique and expertise. Lab. Anim. 32, 377–386 (1998).
McGuill, M.W. & Rowan, A.N. Biological effects of blood loss: implications for sampling volumes and techniques. ILAR News 31, 5–20 (1989).
Removal of blood from laboratory mammals and birds. First report of the BVA/FRAME/RSPCA/UFAW Joint Working Group on Refinement. Lab. Anim. 27, 1–22 (1993).
Acknowledgements
We thank D. Lebeaux for critical reading of the manuscript. We thank R. Ramphal and P. Courvalin for providing strains. This work was supported by the French government's Investissement d'Avenir program, Laboratoire d'Excellence 'Integrative Biology of Emerging Infectious Diseases' (grant ANR-10-LABX-62-IBEID) and the Fondation pour la 'Recherche Médicale grant' (Equipe FRM DEQ20140329508).
Author information
Authors and Affiliations
Contributions
A.C. and C.B. initiated the development of the model. A.C., J.-M.G. and C.B. designed the experiments and wrote the manuscript. A.C. performed the experiments.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Biofilm led to lethal infection in immunosuppressed rats.
(a) TIVAP implanted and cyclophosphamide-treated rats (see Supplementary Method 1 for detailed procedure) were injected with 102 CFU in 100µL of P. aeruginosa into the port of TIVAP at day +4 (4 days after TIVAP implantation) and photon emission was monitored for 3 days to evaluate biofilm formation and associated infection. (b) Bacterial load from different organs aseptically removed on day 3 post infection from dead animals was analyzed, organs were homogenized and were plated on LB agar for viable counts per mL. CFU results are means +/- standard deviations. Number of rats (n) used in the experiment, n = 4. Panel b is a modified version of Figure S5B and C from ref. 31 (Chauhan, A. et al., Antimicrob. Agents Chemother. 56, 6310–6318, 2012) and is adapted with permission from the American Society for Microbiology. Work on animals was performed in compliance with French and European regulations on care and protection of laboratory animals (European Commission directive 2010/63; French law 2013-118, 06th February, 2013). The protocols used in this study were approved by the ethics committee of “Paris Centre et Sud N°59” (reference 2012-0045).
Supplementary Figure 2 In vivo lock therapy against Staphylococcus aureus biofilm in the implanted TIVAP: 5-day regimen presented.
200 µL high dose antibiotics solution was instilled in TIVAP of rats (day +4) to treat methicillin sensitive S. aureus biofilm colonization (n = 5). Lock therapy was associated with systemic vancomycin for S. aureus. The lock was renewed every 24 h and 4 times (up to day +8), and its efficacy was monitored as photon emissions. See Supplementary Method 2 for detailed procedure. (a) Untreated control rats with 1X PBS lock. (b) 5 mg/mL gentamicin lock. (c) 30 mg/mL EDTA alone. (d) Combined gentamicin (5 mg/mL) and EDTA (30 mg/mL) lock. In panels (a) to (d), representative experiments are shown. (e-h) Rats were euthanized 7 days after the last lock instillation, (day +15) and TIVAP were harvested and monitored for photon emissions. (e) Untreated control 1X PBS lock, (f) 5 mg/mL gentamicin lock, (g) 30 mg/mL EDTA alone and (h) Combined gentamicin (5 mg/mL) and EDTA (30 mg/mL) lock. (i) Bacterial cells from TIVAP were harvested and plated on TSB agar for counts of CFU/mL. CFU results are means +/- standard deviations. One-way analysis of variance (ANOVA) with Graphpad Prism version 5.0c was used for statistical analysis. A P value of <0.05 was considered significant, **** P <0.0001. Panel i is a modified version of Figure 1G from ref. 31 (Chauhan, A. et al., Antimicrob. Agents Chemother. 56, 6310–6318, 2012) and is adapted with permission from the American Society for Microbiology, and is reprinted with authorization. Work on animals was performed in compliance with French and European regulations on care and protection of laboratory animals (European Commission directive 2010/63; French law 2013-118, 06th February, 2013). The protocols used in this study were approved by the ethics committee of “Paris Centre et Sud N°59” (reference 2012-0045).
Supplementary Figure 3 Modified anti-adhesive totally implantable venous access port (TIVAP).
(a) Commercially available pediatric TIVAP used in the study, and dismantled TIVAP (1, silicone catheter; 2 and 3, envelope of port; 4, sealing ring of port; 5, silicone septum; 6, titanium port) and anti-adhesive molecules used to modify TIVAP parts. The silicone catheter and septum were modified using methylcellulose (MeCe) derivative and the titanium port was modified using polyethylene glycol (PEG) derivative. (b) Rats with modified or unmodified implanted TIVAPs were inoculated with 106 colony-forming units (CFUs) of Staphylococcus aureus or 103 CFUs of Pseudomonas aeruginosa per 50μL of 1X phosphate-buffered saline. Bacteria were allowed to adhere to the TIVAP endoluminal surface for 3 hours (S. aureus) or 1.5 hours (P. aeruginosa) and biofilms were left to form for 5 days, and TIVAP was extracted to measure bacterial biofilm colonization. Viable bacteria were counted by plating on tryptic soy agar for S. aureus or lysogeny broth agar for P. aeruginosa. (Control: unmodified TIVAP. Si-Ti: TIVAP with silicone catheter and septum, and titanium modified parts as detailed in panel (a)). Statistical analysis was performed using 1-way analysis of variance (ANOVA) with GraphPad Prism software (version 5.0c). Differences were considered significant at P < 0.05. *P ≤ 0.01; **P ≤ 0.001. Panels (a) and (b) are reprinted from, respectively, Figure 1B and 3B from ref. 29 (Chauhan, A. et al., J. Infect. Dis. 210, 1347–1356, 2014) and is adapted with permission from Oxford University Press and the Infectious Diseases Society of America. Work on animals was performed in compliance with French and European regulations on care and protection of laboratory animals (European Commission directive 2010/63; French law 2013-118, 06th February, 2013). The protocols used in this study were approved by the ethics committee of “Paris Centre et Sud N°59” (reference 2012-0045).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3, Supplementary Methods 1–4 and Supplementary Table 1 (PDF 797 kb)
Rights and permissions
About this article
Cite this article
Chauhan, A., Ghigo, JM. & Beloin, C. Study of in vivo catheter biofilm infections using pediatric central venous catheter implanted in rat. Nat Protoc 11, 525–541 (2016). https://doi.org/10.1038/nprot.2016.033
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2016.033
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.