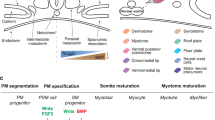
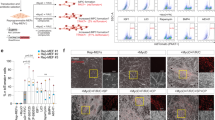
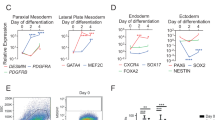
Abstract
Skeletal muscle is the most abundant human tissue; therefore, an unlimited availability of myogenic cells has applications in regenerative medicine and drug development. Here we detail a protocol to derive myogenic cells from human embryonic stem (ES) and induced pluripotent stem (iPS) cells, and we also provide evidence for its extension to human iPS cells cultured without feeder cells. The procedure, which does not require the generation of embryoid bodies or prospective cell isolation, entails four stages with different culture densities, media and surface coating. Pluripotent stem cells are disaggregated to single cells and then differentiated into expandable cells resembling human mesoangioblasts. Subsequently, transient Myod1 induction efficiently drives myogenic differentiation into multinucleated myotubes. Cells derived from patients with muscular dystrophy and differentiated using this protocol have been genetically corrected, and they were proven to have therapeutic potential in dystrophic mice. Thus, this platform has been demonstrated to be amenable to gene and cell therapy, and it could be extended to muscle tissue engineering and disease modeling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
05 August 2015
In the version of this article initially published, the concentration of ROCK inhibitor Y27362 in Step 2 was incorrectly given as 10 mM. The correct concentration to be used is 10 µM. The error has been corrected in the HTML and PDF versions of the article.
References
Mercuri, E. & Muntoni, F. Muscular dystrophies. Lancet 381, 845–860 (2013).
Benedetti, S., Hoshiya, H. & Tedesco, F.S. Repair or replace? Exploiting novel gene and cell therapy strategies for muscular dystrophies. FEBS J. 280, 4263–4280 (2013).
Fairclough, R.J., Perkins, K.J. & Davies, K.E. Pharmacologically targeting the primary defect and downstream pathology in Duchenne muscular dystrophy. Curr. Gene Ther. 12, 206–244 (2012).
Perie, S. et al. Autologous myoblast transplantation for oculopharyngeal muscular dystrophy: a phase I/IIa clinical study. Mol. Ther. 22, 219–225 (2014).
Dellavalle, A. et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat. Cell Biol. 9, 255–267 (2007).
Sacco, A. et al. Short telomeres and stem cell exhaustion model Duchenne muscular dystrophy in mdx/mTR mice. Cell 143, 1059–1071 (2010).
Blau, H.M., Webster, C. & Pavlath, G.K. Defective myoblasts identified in Duchenne muscular dystrophy. Proc. Natl. Acad. Sci. USA 80, 4856–4860 (1983).
Cohn, R.D. et al. Disruption of DAG1 in differentiated skeletal muscle reveals a role for dystroglycan in muscle regeneration. Cell 110, 639–648 (2002).
Kudryashova, E., Kramerova, I. & Spencer, M.J. Satellite cell senescence underlies myopathy in a mouse model of limb-girdle muscular dystrophy 2H. J. Clin. Invest. 122, 1764–1776 (2012).
Cassano, M. et al. α-sarcoglycan is required for FGF-dependent myogenic progenitor cell proliferation in vitro and in vivo. Development 138, 4523–4533 (2011).
Tedesco, F.S. et al. Transplantation of genetically corrected human iPSC-derived progenitors in mice with limb-girdle muscular dystrophy. Sci. Transl. Med. 4, 140ra189 (2012).
Yamanaka, S. Induced pluripotent stem cells: past, present, and future. Cell Stem Cell 10, 678–684 (2012).
Gerli, M.F., Maffioletti, S.M., Millet, Q. & Tedesco, F.S. Transplantation of induced pluripotent stem cell-derived mesoangioblast-like myogenic progenitors in mouse models of muscle regeneration. J. Vis. Exp. e50532 (2014).
Li, O. et al. Human iPSC-derived mesoangioblasts, like their tissue-derived counterparts, suppress T cell proliferation through IDO- and PGE-2-dependent pathways. F1000 Res. 2, 24 (2013).
Goldberg-Cohen, I., Beck, G., Ziskind, A. & Itskovitz-Eldor, J. Vascular cells. Methods Enzymol. 418, 252–266 (2006).
Dey, J. et al. MyoD is a tumor suppressor gene in medulloblastoma. Cancer Res. 73, 6828–6837 (2013).
Rideout, W.M. III et al. Progressive increases in the methylation status and heterochromatinization of the myoD CpG island during oncogenic transformation. Mol. Cell. Biol. 14, 6143–6152 (1994).
Ferreira, L.M. & Mostajo-Radji, M.A. How induced pluripotent stem cells are redefining personalized medicine. Gene 520, 1–6 (2013).
Barberi, T. et al. Derivation of engraftable skeletal myoblasts from human embryonic stem cells. Nat. Med. 13, 642–648 (2007).
Darabi, R. et al. Human ES- and iPS-derived myogenic progenitors restore DYSTROPHIN and improve contractility upon transplantation in dystrophic mice. Cell Stem Cell 10, 610–619 (2012).
Awaya, T. et al. Selective development of myogenic mesenchymal cells from human embryonic and induced pluripotent stem cells. PLoS ONE 7, e51638 (2012).
Goudenege, S. et al. Myoblasts derived from normal hESCs and dystrophic hiPSCs efficiently fuse with existing muscle fibers following transplantation. Mol. Ther. 20, 2153–2167 (2012).
Tanaka, A. et al. Efficient and reproducible myogenic differentiation from human iPS cells: prospects for modeling Miyoshi myopathy. PLoS ONE 8, e61540 (2013).
Abujarour, R. et al. Myogenic differentiation of muscular dystrophy-specific induced pluripotent stem cells for use in drug discovery. Stem Cells Transl. Med. 3, 149–160 (2014).
Borchin, B., Chen, J. & Barberi, T. Derivation and FACS-mediated purification of PAX3+/PAX7+ skeletal muscle precursors from human pluripotent stem cells. Stem Cell Rep. 1, 620–631 (2013).
Xu, C. et al. A zebrafish embryo culture system defines factors that promote vertebrate myogenesis across species. Cell 155, 909–921 (2013).
Shelton, M. et al. Derivation and expansion of PAX7-positive muscle progenitors from human and mouse embryonic stem cells. Stem Cell Reports 3, 516–529 (2014).
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007).
Aflatoonian, B. et al. Generation of Sheffield (Shef) human embryonic stem cell lines using a microdrop culture system. In vitro Cell. Dev. Biol. Anim. 46, 236–241 (2010).
Watanabe, K. et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat. Biotechnol. 25, 681–686 (2007).
Sanes, J.R. The basement membrane/basal lamina of skeletal muscle. J. Biol. Chem. 278, 12601–12604 (2003).
Malinda, K.M. Matrigel migration and angiogenesis assay. Methods Mol. Biol. 467, 287–294 (2009).
Ponce, M.L. Tube formation: an in vitro Matrigel angiogenesis assay. Methods Mol. Biol. 467, 183–188 (2009).
Grefte, S., Vullinghs, S., Kuijpers-Jagtman, A.M., Torensma, R. & Von den Hoff, J.W. Matrigel, but not collagen I, maintains the differentiation capacity of muscle derived cells. Biomed. Mater. 7, 055004 (2012).
Kuhl, U., Timpl, R. & von der Mark, K. Synthesis of type IV collagen and laminin in cultures of skeletal muscle cells and their assembly on the surface of myotubes. Dev. Biol. 93, 344–354 (1982).
Yin, H., Price, F. & Rudnicki, M.A. Satellite cells and the muscle stem cell niche. Physiol. Rev. 93, 23–67 (2013).
Tonlorenzi, R., Dellavalle, A., Schnapp, E., Cossu, G. & Sampaolesi, M. Isolation and characterization of mesoangioblasts from mouse, dog, and human tissues. Curr. Protoc. Stem Cell Biol. Chapter 2 Unit 2B1 (2007).
Tedesco, F.S. et al. Stem cell–mediated transfer of a human artificial chromosome ameliorates muscular dystrophy. Sci. Transl. Med. 3, 96ra78 (2011).
Kimura, E. et al. Cell-lineage regulated myogenesis for dystrophin replacement: a novel therapeutic approach for treatment of muscular dystrophy. Hum. Mol. Genet. 17, 2507–2517 (2008).
Dorchies, O.M. et al. The anticancer drug tamoxifen counteracts the pathology in a mouse model of Duchenne muscular dystrophy. Am. J. Pathol. 182, 485–504 (2013).
Nakano-Okuno, M., Borah, B.R. & Nakano, I. Ethics of iPSC-based clinical research for age-related macular degeneration: patient-centered risk-benefit analysis. Stem Cell Rev. 10, 743–752 (2014).
Cyranoski, D. Japanese woman is first recipient of next-generation stem cells. Nature doi:10.1038/nature.2014.15915 (12 September2014).
Bejjani, B.A. & Shaffer, L.G. Application of array-based comparative genomic hybridization to clinical diagnostics. J. Mol. Diagn. 8, 528–533 (2006).
Workman, P. et al. Guidelines for the welfare and use of animals in cancer research. Br. J. Cancer 102, 1555–1577 (2010).
Montini, E. et al. The genotoxic potential of retroviral vectors is strongly modulated by vector design and integration site selection in a mouse model of HSC gene therapy. J. Clin. Invest. 119, 964–975 (2009).
Aiuti, A. et al. Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome. Science 341, 1233151 (2013).
Biffi, A. et al. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science 341, 1233158 (2013).
Matrai, J. et al. Hepatocyte-targeted expression by integrase-defective lentiviral vectors induces antigen-specific tolerance in mice with low genotoxic risk. Hepatology 53, 1696–1707 (2011).
Matrai, J., Chuah, M.K. & VandenDriessche, T. Recent advances in lentiviral vector development and applications. Mol. Ther. 18, 477–490 (2010).
Oshimura, M., Uno, N., Kazuki, Y., Katoh, M. & Inoue, T. A pathway from chromosome transfer to engineering resulting in human and mouse artificial chromosomes for a variety of applications to bio-medical challenges. Chromosome Res. 23, 111–133 (2015).
Tedesco, F.S. Human artificial chromosomes for Duchenne muscular dystrophy and beyond: challenges and hopes. Chromosome Res. 23, 135–141 (2015).
Follenzi, A. & Naldini, L. Generation of HIV-1–derived lentiviral vectors. Methods Enzymol. 346, 454–465 (2002).
Acknowledgements
We thank G. Cossu (University of Manchester, UK) and all co-authors of the original article describing this technology for their initial support and contribution. We are also grateful to the following biobanks and colleagues for some of the cells used in this study and/or in its relative original article: (i) P. Andrews, H. Moore (University of Sheffield, UK) and the UK Stem Cell Bank for kindly supplying Shef 6 human ES cells; (ii) M. Mora and The 'Cells, tissues and DNA from patients with neuromuscular diseases Biobank', member of the Telethon Network of Genetic Biobanks (project no. GTB12001), funded by Telethon Italy and of the EuroBioBank network; (iii) B. Schoser, P. Schneiderat and The Muscle Tissue Culture Collection, part of the German network on muscular dystrophies (service structure S1, 01GM0601) and the German network for mitochondrial disorders (project D2, 01GM0862) funded by the German ministry of education and research (BMBF, Bonn, Germany). The Muscle Tissue Culture Collection is a partner of EuroBioBank (http://www.eurobiobank.org) and TREAT-NMD (http://www.treat-nmd.eu); (iv) Y. Torrente, M. Moggio and the Telethon Network of Genetic Biobanks at Ospedale Maggiore Policlinico, Milan, Italy; (v) M. Oshimura and Y. Kazuki (the Tottori University, Yonago, Japan) for the DMD iPS cells (generated from Coriell Institute fibroblasts GM05169, US National Institute of General Medical Sciences (NIGMS) Human Genetic Cell Repository). We also thank J. Chamberlain (University of Washington, Seattle, WA) for the original MyoD-ER(T) construct. This project has received funding from the European Union's Seventh Framework Programme for research, technological development and demonstration under grant agreement no. 602423 (PluriMes) and from Takeda New Frontier Science program. Work in the Tedesco laboratory is also supported by the UK Medical Research Council (MRC) and the Biotechnology and Biological Sciences Research Council (BBSRC), Duchenne Parent Project Onlus, Muscular Dystrophy UK, Duchenne Children's Trust and the Duchenne Research Fund. M.K.C., T.V.D., S.D. and M.L. were supported by the Fund for Scientific Research (FWO), Association Française contre les Myopathies and the Willy Gepts Fund (VUB).
AUTHOR CONTRIBUTIONS
S.M.M. and M.F.M.G. performed the experiments with the help of M.R., analyzed the data and wrote the manuscript; S.D. performed experiments on feeder-free iPS cells and HIDEMs, and discussed and analyzed results with T.V.D. and M.K.C.; S.B. performed the proliferation assay and analyzed and discussed results; M.L. performed live imaging experiments and analyzed and discussed results; S.D. and M.L. performed most of the work described here in a collaborative project as visiting PhD students at University College London; F.S.T. developed the protocol, discussed and analyzed the results with all coauthors, and wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The work described in this article is partially covered by patent application no. PCT/GB2013/050112 (publication no. WO2013108039 A1). F.S.T. is the principal investigator of a grant funded by the Takeda New Frontier Science program and provided speaking and consulting services to Takeda Pharmaceuticals International, Inc. via UCL Consultants. The remaining authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Additional cell characterization: pluripotency analysis and alkaline phosphatase staining.
(a) The histogram on the left-hand side shows absence of alkaline phosphatase (AP) signal in one HIDEM line, whereas the picture on the right-hand side shows AP activity detected with enzymatic reaction (purple/black precipitate; scale bar 50 μm). (b) Representative immunofluorescence staining for the pluripotency factors NANOG, OCT4 and SOX2. Feeder-free HIDEMs (FF HIDEMs) are negative for all the markers and are shown in the left part of the panel. On the right hand side, stained feeder-free iPS (FF hiPS) cells are shown as positive control (scale bar 50 μm).
Supplementary information
Supplementary Text and Figures
Supplementary Figure 1, Supplementary Tables 1 and 2, Supplementary Methods (PDF 264 kb)
The video shows a myotube derived from differentiated DMD HIDEMs twitching in culture at day 8 in myogenic differentiation medium.
Sarcomeric striations are visible in their characteristic structure of alternate dark and light bands (640X magnification). (MP4 17149 kb)
Source data
Rights and permissions
About this article
Cite this article
Maffioletti, S., Gerli, M., Ragazzi, M. et al. Efficient derivation and inducible differentiation of expandable skeletal myogenic cells from human ES and patient-specific iPS cells. Nat Protoc 10, 941–958 (2015). https://doi.org/10.1038/nprot.2015.057
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2015.057
This article is cited by
-
3D human induced pluripotent stem cell–derived bioengineered skeletal muscles for tissue, disease and therapy modeling
Nature Protocols (2023)
-
Simple and efficient differentiation of human iPSCs into contractible skeletal muscles for muscular disease modeling
Scientific Reports (2023)
-
Control of satellite cell function in muscle regeneration and its disruption in ageing
Nature Reviews Molecular Cell Biology (2022)
-
Acute conversion of patient-derived Duchenne muscular dystrophy iPSC into myotubes reveals constitutive and inducible over-activation of TGFβ-dependent pro-fibrotic signaling
Skeletal Muscle (2020)
-
Abnormal nuclear aggregation and myotube degeneration in myotonic dystrophy type 1
Neurological Sciences (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.