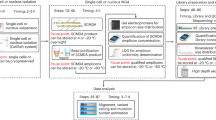
Abstract
We describe a sensitive, robust, high-throughput method for quantifying the formation of micronuclei, markers of genome instability, in mouse erythrocytes. Micronuclei are whole chromosomes or chromosome segments that have been separated from the nucleus. Other methods of detection rely on labor-intensive, microscopy-based techniques. Here we describe a 2-d, 96-well plate–based flow cytometric method of micronucleus scoring that is simple enough for a research technician experienced in flow cytometry to perform. The assay detects low levels of genome instability that cannot be readily identified by classic phenotyping, using 25 μl of blood. By using this assay, we have screened >10,000 blood samples and discovered novel genes that contribute to vertebrate genome maintenance, as well as novel disease models and mechanisms of genome instability disorders. We discuss experimental design considerations, including statistical power calculation, we provide troubleshooting tips and we discuss factors that contribute to a false-positive increase in the number of micronucleated red blood cells and to experimental variability.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Fenech, M. et al. Molecular mechanisms of micronucleus, nucleoplasmic bridge and nuclear bud formation in mammalian and human cells. Mutagenesis 26, 125–132 (2011).
Evans, H.J., Neary, G.J. & Williamson, F.S. The relative biological efficiency of single doses of fast neutrons and gamma-rays on Vicia faba roots and the effect of oxygen. Part II. Chromosone damage: the production of micronuclei. Int. J. Radiat. Biol. 1, 216–229 (1959).
Maluf, S.W. & Erdtmann, B. Genomic instability in Down syndrome and Fanconi anemia assessed by micronucleus analysis and single-cell gel electrophoresis. Cancer Genet. Cytogenet. 124, 71–75 (2001).
Holland, N. et al. The micronucleus assay in human buccal cells as a tool for biomonitoring DNA damage: the HUMN project perspective on current status and knowledge gaps. Mutat. Res. 659, 93–108 (2008).
Hanahan, D. & Weinberg, R.A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Stephens, P.J. et al. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell 144, 27–40 (2011).
Crasta, K. et al. DNA breaks and chromosome pulverization from errors in mitosis. Nature 482, 53–58 (2012).
Hatch, E.M., Fischer, A.H., Deerinck, T.J. & Hetzer, M.W. Catastrophic nuclear envelope collapse in cancer cell micronuclei. Cell 154, 47–60 (2013).
Zhang, C.Z., Leibowitz, M.L. & Pellman, D. Chromothripsis and beyond: rapid genome evolution from complex chromosomal rearrangements. Genes Dev. 27, 2513–2530 (2013).
Forment, J.V., Kaidi, A. & Jackson, S.P. Chromothripsis and cancer: causes and consequences of chromosome shattering. Nat. Rev. Cancer 12, 663–670 (2012).
Crossan, G.P. et al. Disruption of mouse Slx4, a regulator of structure-specific nucleases, phenocopies Fanconi anemia. Nat. Genet. 43, 147–152 (2011).
Shima, N. et al. A viable allele of Mcm4 causes chromosome instability and mammary adenocarcinomas in mice. Nat. Genet. 39, 93–98 (2007).
Hodskinson, M.R. et al. Mouse SLX4 is a tumor suppressor that stimulates the activity of the nuclease XPF-ERCC1 in DNA crosslink repair. Mol. Cell 54, 472–484 (2014).
Nakachi, K., Hayashi, T., Hamatani, K., Eguchi, H. & Kusunoki, Y. Sixty years of follow-up of Hiroshima and Nagasaki survivors: current progress in molecular epidemiology studies. Mutat. Res. 659, 109–117 (2008).
Doherty, A.T. The in vitro micronucleus assay. Methods Mol. Biol. 817, 121–141 (2012).
Liu, L., Liu, Y., Ni, G. & Liu, S. Flow cytometric scoring of micronucleated reticulocytes as a possible high-throughput radiation biodosimeter. Environ. Mol. Mutagen. 51, 215–221 (2010).
Maluf, S.W., Passos, D.F., Bacelar, A., Speit, G. & Erdtmann, B. Assessment of DNA damage in lymphocytes of workers exposed to X-radiation using the micronucleus test and the comet assay. Environ. Mol. Mutagen. 38, 311–315 (2001).
Fliedner, T.M., Andrews, G.A., Cronkite, E.P. & Bond, V.P. Early and late cytologic effects of whole body irradiation on human marrow. Blood 23, 471–487 (1964).
Howell, W.H. The life history of the formed elements of the blood, especially the red blood corpuscles. J. Morphol. 4, 57–116 (1891).
Jolly, J.M.J. Sur la formation des globules rouges des mammifères. Compt. Rend. de la Société de Biol. 58, 528–531 (1905).
Countryman, P.I. & Heddle, J.A. The production of micronuclei from chromosome aberrations in irradiated cultures of human lymphocytes. Mutat. Res. 41, 321–332 (1976).
Sears, D.A. & Udden, M.M. Howell-Jolly bodies: a brief historical review. Am. J. Med. Sci. 343, 407–409 (2012).
MacGregor, J.T., Wehr, C.M. & Gould, D.H. Clastogen-induced micronuclei in peripheral blood erythrocytes: the basis of an improved micronucleus test. Environ. Mutagen. 2, 509–514 (1980).
Hutter, K.J. & Stohr, M. Rapid detection of mutagen induced micronucleated erythrocytes by flow cytometry. Histochemistry 75, 353–362 (1982).
Grawe, J., Zetterberg, G. & Amneus, H. Flow-cytometric enumeration of micronucleated polychromatic erythrocytes in mouse peripheral blood. Cytometry 13, 750–758 (1992).
Hayashi, M., Norppa, H., Sofuni, T. & Ishidate, M. Jr. Flow cytometric micronucleus test with mouse peripheral erythrocytes. Mutagenesis 7, 257–264 (1992).
Lee, L.G., Chen, C.H. & Chiu, L.A. Thiazole orange: a new dye for reticulocyte analysis. Cytometry 7, 508–517 (1986).
Bender, J.G. et al. Identification and comparison of CD34-positive cells and their subpopulations from normal peripheral blood and bone marrow using multicolor flow cytometry. Blood 77, 2591–2596 (1991).
Dertinger, S.D., Torous, D.K. & Tometsko, K.R. Simple and reliable enumeration of micronucleated reticulocytes with a single-laser flow cytometer. Mutat. Res. 371, 283–292 (1996).
Dertinger, S.D., Torous, D.K., Hayashi, M. & MacGregor, J.T. Flow cytometric scoring of micronucleated erythrocytes: an efficient platform for assessing in vivo cytogenetic damage. Mutagenesis 26, 139–145 (2011).
Reinholdt, L., Ashley, T., Schimenti, J. & Shima, N. Forward genetic screens for meiotic and mitotic recombination-defective mutants in mice. Methods Mol. Biol. 262, 87–107 (2004).
Shima, N. et al. Phenotype-based identification of mouse chromosome instability mutants. Genetics 163, 1031–1040 (2003).
White, J.K. et al. Genome-wide generation and systematic phenotyping of knockout mice reveals new roles for many genes. Cell 154, 452–464 (2013).
Chen, J. et al. Mcph1-deficient mice reveal a role for MCPH1 in otitis media. PLoS ONE 8, e58156 (2013).
McIntyre, R.E. et al. Disruption of mouse Cenpj, a regulator of centriole biogenesis, phenocopies Seckel syndrome. PLoS Genet. 8, e1003022 (2012).
Nijnik, A. et al. The critical role of histone H2A-deubiquitinase Mysm1 in hematopoiesis and lymphocyte differentiation. Blood 119, 1370–1379 (2012).
Balmus, G. et al. Disease severity in a mouse model of ataxia telangiectasia is modulated by the DNA damage checkpoint gene Hus1. Hum. Mol. Genet. 21, 3408–3420 (2012).
Holloway, J.K. et al. Mammalian BTBD12 (SLX4) protects against genomic instability during mammalian spermatogenesis. PLoS Genet. 7, e1002094 (2011).
Dertinger, S.D. et al. Enumeration of micronucleated CD71-positive human reticulocytes with a single-laser flow cytometer. Mutat. Res. 515, 3–14 (2002).
Fenech, M. Cytokinesis-block micronucleus cytome assay. Nat. Protoc. 2, 1084–1104 (2007).
Danise, P. et al. Evaluation of nucleated red blood cells in the peripheral blood of hematological diseases. Clin. Chem. Lab. Med. 50, 357–360 (2012).
Balmus, G. & McIntyre, R.E. Genetic screens in mice for genome integrity maintenance and cancer predisposition. Curr. Opin. Genet. Dev. 24, 1–7 (2014).
Kilkenny, C., Browne, W.J., Cuthill, I.C., Emerson, M. & Altman, D.G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 8, e1000412 (2010).
Parasuraman, S., Raveendran, R. & Kesavan, R. Blood sample collection in small laboratory animals. J. Pharmacol. Pharmacother. 1, 87–93 (2010).
Finkel, T., Serrano, M. & Blasco, M.A. The common biology of cancer and ageing. Nature 448, 767–774 (2007).
Karp, N.A., Melvin, D., Sanger Mouse Genetics Project & Mott, R.F. Robust and sensitive analysis of mouse knockout phenotypes. PLoS ONE 7, e52410 (2012).
Lenth, R.V. Statistical power calculations. J. Anim. Sci. 85, E24–E29 (2007).
Acknowledgements
We thank M. Hitcham and N. Harman for assistance with blood collections, W. Cheng for assistance with flow cytometry during high-throughput screening and K. Dry for comments on the manuscript. R.E.M. is supported by Cancer Research UK (CRUK; project grant C20510/A12401). D.J.A. is supported by CRUK. D.J.A. and B.L.N. are supported by the Wellcome Trust. Research in the Jackson Laboratory is funded by CRUK program grant no. C6/A11224, the European Research Council and the European Community Seventh Framework Programme grant agreement no. HEALTH-F2-2010-259893 (DDResponse). Core funding is provided by CRUK (C6946/A14492) and the Wellcome Trust (WT092096). S.P.J. receives his salary from the University of Cambridge, UK, supplemented by CRUK. G.B. is funded by CRUK program grant no. C6/A11224.
Author information
Authors and Affiliations
Contributions
D.J.A. and S.P.J. conceived the idea of screening mice from large-scale mouse phenotyping pipelines for micronucleated erythrocytes. G.B. and R.E.M. performed the experimental analysis and wrote the manuscript with comments from all authors. B.L.N. assisted with flow cytometry and N.A.K. performed statistical analysis.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Severely increased red blood cell production (reticulocytosis) results in detection of nucleated and micronucleated (MN) normochromatic erythrocytes (NCE) in Q3.
Blood obtained from C57BL/6J-ApcMin/+ mice was processed according to the methods described herein. a) Representative flow-cytometric analysis for six week old Apc-/min mouse (left) and 16 week old Apcmin/+ mice (right). Note the change in % reticulocytes (RET) and %MN-NCE. During extremely high rates of erythropoiesis, such as that exhibited by severely anaemic, aged Apcmin/+ mice (right), the number of reticulocytes detected in the RET quadrant is vastly increased, however the process becomes error prone and the nucleus is not always expelled; both nucleated and micronucleated red blood cells are detected in the % MN-NCE quadrant, leading to a false-positive increase in %MN-NCE. b) Data shows %MN-NCE vs. %RET for Apcmin/+ mice (n=4) and Apc+/+ mice (n=10). Linear regression analysis shows that there is a significant correlation between increased % MN-NCE and % RET (P<0.01). c) Dot plot showing the average %RET in male C57BL/6 mice (mean ± standard deviation: 1.623±0.209; n=20).
Supplementary Figure 2 Effect of RNase, flow rate and fixation.
a) Blood sample obtained from male C57BL/6 mouse at 16 weeks of age, processed according to the methods described herein. b) The same sample was processed without the addition of RNase, which results in a shift of the FITC-conjugated transferrin receptor-positive (CD71-FITC+), propidium iodide negative (PI-) reticulocyte (RET) population over to the micronucleated-RET (MN-RET) quadrant. This is caused by PI binding to the RNA within the CD71-FITC+ RETs. c) Sample analyzed at a rate of 10000 events per second (vs. 1000 events in a) shows spikes of events in the CD71-FITC+ and PI+ quadrant (indicated by arrow head). d) The color of fixed samples can indicate whether samples have become too warm during preparation. Fresh blood samples were harvested and fixed in -80°C methanol in duplicate or in room temperature (RT; 20-25°C) methanol. The blood fixed in -80°C methanol shows characteristic red color while the sample fixed in RT methanol shows brown color. e) Samples maintained for long periods of time in -80°C methanol show characteristic red color and are suitable for processing. f) The same samples (from image d) were either placed in a -80°C freezer or left at RT. After 30 minutes the sample that was maintained at -80°C showed the characteristic red color while the sample that was left at RT became brown in color; no detectable change in color was seen at the sample fixed in RT methanol.
Supplementary Figure 3 Indicators of incorrect sample processing.
a) Correctly prepared sample. Blood sample obtained from male C57BL/6 mouse at 16 weeks of age, processed according to the methods described herein. The SSC-A vs. FSC-A plot (left) reveals correct separation of blood cell populations and minimal cell debris and the CD71-FITC vs. PI plot (right) reveals correct representation of populations within the quadrant gates. b) Slightly degraded blood sample. Blood sample left 5 days at 4˚C and fixed in -80˚C methanol. The SSC-A vs. FSC-A plot (left) reveals increased cell debris (black arrow) and incomplete separation of the cell populations. The gated populations plotted on CD71-FITC vs. PI plot (right) show no indication of degradation but there is a slight increase in MN-NCE (right). c) Very degraded blood sample. Blood sample left 3 weeks at 4˚C and fixed in -80˚C methanol. Both the SSC-A vs. FSC-A and CD71-FITC vs. PI plots are completely transformed. d) Blood sample fixed in warm methanol (room-temperature). The SSC-A vs. FSC-A plot (left) reveals increased cell debris (black arrow) and the CD71-FITC vs. PI plot (right) shows incorrect representation of populations. e) Blood sample fixed correctly (-80˚C methanol) but allowed to warm to room temperature (20-25°C) before processing. The SSC-A vs. FSC-A plot (left) reveals increased cell debris (black arrow) and incomplete separation of the cell populations, and the CD71-FITC vs. PI plot (right) shows incorrect representation of populations.
Supplementary Figure 4 Effect of incubation time in propidium iodide (PI) and time in storage.
a) Data shows %MN-NCE for replicate tubes of the same sample that were stored at -80˚C. Two tubes were processed at monthly intervals over a one-year period. Linear regression analysis shows that there is no significant difference in the detection of % MN-NCE up to one year after fixation (P=0.427). b) A single blood sample was added to each well of a 96-well plate and processed accordingly. After washing with buffer to remove the FITC-conjugated transferrin receptor (CD71-FITC)/RNase mix, samples were resuspended in buffer containing 1 mg/ml PI, and all 96 wells were immediately analyzed by flow-cytometry. Linear regression analysis shows that there is a statistically significant difference in the % micronucleated (MN)-normochromatic erythrocytes (NCE) obtained at the beginning of the plate versus those obtained at the end of the plate (*P<0.001). This could be due to saturation of DNA with PI over time. The biological effect is small but to avoid potential experimental bias, samples should be randomized across the plate and we recommend adding PI to 24 wells at a time.
Supplementary Figure 5 Flow-cytometric analysis of control samples and gating out autofluorescence.
Unstained samples are used to centralise the main cell population within the side scatter (SSC) versus forward scatter (FSC) plot (data not shown). The main population is gated (erythrocytes), and within the propidium iodide (PI) versus FITC-conjugated transferrin receptor (CD71-FITC) plot a quadrant gate is drawn. Single-stain controls are then analysed to determine the correct voltage and gain settings, which ensures the majority of events fall within the scale of the X and Y axes, and within the correct quadrant gate. a) Unstained control sample and b) CD71-FITC alone. Auto-fluorescence can be detected with samples stained with PI alone, either within the transferrin receptor FITC-conjugated (CD71-FITC) channel (c; indicated by arrowhead), or within the higher logarithmic scale (Log) of PI channel (d; indicated by arrowhead). To remove the effect of auto-fluorescence, Region gate R3 is created within the PI versus side scatter (SSC) plot (d).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 (PDF 1199 kb)
Rights and permissions
About this article
Cite this article
Balmus, G., Karp, N., Ng, B. et al. A high-throughput in vivo micronucleus assay for genome instability screening in mice. Nat Protoc 10, 205–215 (2015). https://doi.org/10.1038/nprot.2015.010
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2015.010
This article is cited by
-
Impacts of horticultural environments on Rhinella arenarum (Anura, Bufonidae) populations: exploring genocytotoxic damage and demographic life history traits
Environmental Science and Pollution Research (2024)
-
Genetic determinants of micronucleus formation in vivo
Nature (2024)
-
Evaluation of mutagenesis, necrosis and apoptosis induced by omeprazole in stomach cells of patients with gastritis
Cancer Cell International (2022)
-
Dysregulated APOBEC3G causes DNA damage and promotes genomic instability in multiple myeloma
Blood Cancer Journal (2021)
-
Inorganic Iron Supplementation Rescues Hematological Insufficiency Even Under Intense Exercise Training in a Mouse Model of Iron Deficiency with Anemia
Biological Trace Element Research (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.