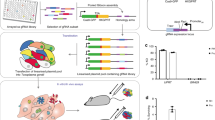
Abstract
The ability to simultaneously assess every gene in a genome for a role in a particular process has obvious appeal. This protocol describes how to perform genome-scale RNAi library screens in bloodstream-form African trypanosomes, a family of parasites that causes lethal human and animal diseases and also serves as a model for studies on basic aspects of eukaryotic biology and evolution. We discuss strain assembly, screen design and implementation, the RNAi target sequencing approach and hit validation, and we provide a step-by-step protocol. A screen can yield from one to thousands of 'hits' associated with the phenotype of interest. The screening protocol itself takes 2 weeks or less to be completed, and high-throughput sequencing may also be completed within weeks. Pre- and post-screen strain assembly, validation and follow-up can take several months, depending on the type of screen and the number of hits analyzed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Alsford, S. et al. High-throughput phenotyping using parallel sequencing of RNA interference targets in the African trypanosome. Genome Res. 21, 915–924 (2011).
Alsford, S. et al. High-throughput decoding of antitrypanosomal drug efficacy and resistance. Nature 482, 232–236 (2012).
Baker, N., Alsford, S. & Horn, D. Genome-wide RNAi screens in African trypanosomes identify the nifurtimox activator NTR and the eflornithine transporter AAT6. Mol. Biochem. Parasitol. 176, 55–57 (2011).
Schumann Burkard, G., Jutzi, P. & Roditi, I. Genome-wide RNAi screens in bloodstream form trypanosomes identify drug transporters. Mol. Biochem. Parasitol. 175, 91–94 (2011).
Schumann Burkard, G. et al. Nucleolar proteins regulate stage-specific gene expression and ribosomal RNA maturation in Trypanosoma brucei. Mol. Microbiol. 88, 827–840 (2013).
Gould, M.K. et al. Cyclic AMP effectors in African trypanosomes revealed by genome-scale RNA interference library screening for resistance to the phosphodiesterase inhibitor CpdA. Antimicrob. Agents Chemother. 57, 4882–4893 (2013).
Mony, B.M. et al. Genome-wide dissection of the quorum sensing signalling pathway in Trypanosoma brucei. Nature 505, 681–685 (2014).
Alsford, S., Currier, R.B., Guerra-Assuncao, J.A., Clark, T.G. & Horn, D. Cathepsin-L can resist lysis by human serum in Trypanosoma brucei brucei. PLoS Pathog. 10, e1004130 (2014).
Hirumi, H. & Hirumi, K. Axenic culture of African trypanosome bloodstream forms. Parasitol. Today 10, 80–84 (1994).
Berriman, M. et al. The genome of the African trypanosome Trypanosoma brucei. Science 309, 416–422 (2005).
Horn, D. Codon usage suggests that translational selection has a major impact on protein expression in trypanosomatids. BMC Genomics 9, 2 (2008).
Jackson, A.P. Tandem gene arrays in Trypanosoma brucei: comparative phylogenomic analysis of duplicate sequence variation. BMC Evol. Biol. 7, 54 (2007).
Carruthers, V.B., van der Ploeg, L.H. & Cross, G.A. DNA-mediated transformation of bloodstream-form Trypanosoma brucei. Nucleic Acids Res. 21, 2537–2538 (1993).
Wirtz, E. & Clayton, C. Inducible gene expression in trypanosomes mediated by a prokaryotic repressor. Science 268, 1179–1183 (1995).
Ngo, H., Tschudi, C., Gull, K. & Ullu, E. Double-stranded RNA induces mRNA degradation in Trypanosoma brucei. Proc. Natl. Acad. Sci. USA 95, 14687–14692 (1998).
LaCount, D.J., Bruse, S., Hill, K.L. & Donelson, J.E. Double-stranded RNA interference in Trypanosoma brucei using head-to-head promoters. Mol. Biochem. Parasitol. 111, 67–76 (2000).
Wang, Z., Morris, J.C., Drew, M.E. & Englund, P.T. Inhibition of Trypanosoma brucei gene expression by RNA interference using an integratable vector with opposing T7 promoters. J. Biol. Chem. 275, 40174–40179 (2000).
Morris, J.C., Wang, Z., Drew, M.E. & Englund, P.T. Glycolysis modulates trypanosome glycoprotein expression as revealed by an RNAi library. EMBO J. 21, 4429–4438 (2002).
Motyka, S.A. & Englund, P.T. RNA interference for analysis of gene function in trypanosomatids. Curr. Opin. Microbiol. 7, 362–368 (2004).
Redmond, S., Vadivelu, J. & Field, M.C. RNAit: an automated web-based tool for the selection of RNAi targets in Trypanosoma brucei. Mol. Biochem. Parasitol. 128, 115–118 (2003).
Subramaniam, C. et al. Chromosome-wide analysis of gene function by RNA interference in the African trypanosome. Eukaryotic Cell 5, 1539–1549 (2006).
Alsford, S., Kawahara, T., Glover, L. & Horn, D. Tagging a T. brucei RRNA locus improves stable transfection efficiency and circumvents inducible expression position effects. Mol. Biochem. Parasitol. 144, 142–148 (2005).
Alsford, S., Glover, L. & Horn, D. Multiplex analysis of RNA interference defects in Trypanosoma brucei. Mol. Biochem. Parasitol. 139, 129–132 (2005).
Glover, L., McCulloch, R. & Horn, D. Sequence homology and microhomology dominate chromosomal double-strand break repair in African trypanosomes. Nucleic Acids Res. 36, 2608–2618 (2008).
Glover, L. & Horn, D. Site-specific DNA double-strand breaks greatly increase stable transformation efficiency in Trypanosoma brucei. Mol. Biochem. Parasitol. 166, 194–197 (2009).
Langridge, G.C. et al. Simultaneous assay of every Salmonella typhi gene using one million transposon mutants. Genome Res. 19, 2308–2316 (2009).
Drew, M.E. et al. The adenosine analog tubercidin inhibits glycolysis in Trypanosoma brucei as revealed by an RNA interference library. J. Biol. Chem. 278, 46596–46600 (2003).
Wilkinson, S.R., Taylor, M.C., Horn, D., Kelly, J.M. & Cheeseman, I. A mechanism for cross-resistance to nifurtimox and benznidazole in trypanosomes. Proc. Natl. Acad. Sci. USA 105, 5022–5027 (2008).
Baker, N. et al. Aquaglyceroporin 2 controls susceptibility to melarsoprol and pentamidine in African trypanosomes. Proc. Natl. Acad. Sci. USA 109, 10996–11001 (2012).
Graf, F.E. et al. Aquaporin 2 mutations in Trypanosoma brucei gambiense field isolates correlate with decreased susceptibility to pentamidine and melarsoprol. PLoS Negl. Trop. Dis. 7, e2475 (2013).
Erben, E.D., Fadda, A., Lueong, S., Hoheisel, J.D. & Clayton, C. A genome-wide tethering screen reveals novel potential post-transcriptional regulators in Trypanosoma brucei. PLoS Pathog. 10, e1004178 (2014).
Lye, L.F. et al. Retention and loss of RNA interference pathways in trypanosomatid protozoans. PLoS Pathog. 6, e1001161 (2010).
Wang, T., Wei, J.J., Sabatini, D.M. & Lander, E.S. Genetic screens in human cells using the CRISPR-Cas9 system. Science 343, 80–84 (2014).
van den Hoff, M.J., Moorman, A.F. & Lamers, W.H. Electroporation in 'intracellular' buffer increases cell survival. Nucleic Acids Res. 20, 2902 (1992).
Alsford, S. & Horn, D. Single-locus targeting constructs for reliable regulated RNAi and transgene expression in Trypanosoma brucei. Mol. Biochem. Parasitol. 161, 76–79 (2008).
Wang, L., Feng, Z., Wang, X. & Zhang, X. DEGseq: an R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 26, 136–138 (2010).
Raz, B., Iten, M., Grether-Buhler, Y., Kaminsky, R. & Brun, R. The Alamar Blue assay to determine drug sensitivity of African trypanosomes (T.b. rhodesiense and T.b. gambiense) in vitro. Acta Tropica 68, 139–147 (1997).
Quail, M.A. et al. A large genome center's improvements to the Illumina sequencing system. Nat. Methods 5, 1005–1010 (2008).
Durand-Dubief, M., Kohl, L. & Bastin, P. Efficiency and specificity of RNA interference generated by intra- and intermolecular double stranded RNA in Trypanosoma brucei. Mol. Biochem. Parasitol. 129, 11–21 (2003).
Akiyoshi, B. & Gull, K. Discovery of unconventional kinetochores in kinetoplastids. Cell 156, 1247–1258 (2014).
Jones, N.G. et al. Regulators of Trypanosoma brucei cell cycle progression and differentiation identified using a kinome-wide RNAi screen. PLoS Pathog. 10, e1003886 (2014).
Kalidas, S. et al. Genetic validation of aminoacyl-tRNA synthetases as drug targets in Trypanosoma brucei. Eukaryotic Cell 13, 504–516 (2014).
Vincent, I.M. et al. A molecular mechanism for eflornithine resistance in African trypanosomes. PLoS Pathog. 6, e1001204 (2010).
Chen, Y., Hung, C.H., Burderer, T. & Lee, G.S. Development of RNA interference revertants in Trypanosoma brucei cell lines generated with a double stranded RNA expression construct driven by two opposing promoters. Mol. Biochem. Parasitol. 126, 275–279 (2003).
Motyka, S.A., Zhao, Z., Gull, K. & Englund, P.T. Integration of pZJM library plasmids into unexpected locations in the Trypanosoma brucei genome. Mol. Biochem. Parasitol. 134, 163–167 (2004).
Langmead, B. & Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Rutherford, K. et al. Artemis: sequence visualization and annotation. Bioinformatics 16, 944–945 (2000).
Acknowledgements
We thank P. Englund, Z. Wang, J. Morris and M. Drew ('the pZJM library team') for the RNAi plasmid library, B. Dujon for the pSCM525 plasmid containing a human-codon–optimized version of the I-SceI gene and S. Obado for contributions to the assembly of the 2T1:T7 T. brucei strain. We also thank B. Brunk and O. Harb, and the GeneDB team for making RIT-seq data available through the GeneDB and TritrypDB databases. The work was funded by grants from The Wellcome Trust; 093010/Z/10/Z (D.H.), 100476 (Strategic Award to Biological Chemistry and Drug Discovery, Dundee), 100320/Z/12/Z (D.H. Senior Investigator Award) and 085775/Z/08/Z (The Wellcome Trust Sanger Institute).
Author information
Authors and Affiliations
Contributions
L.G., S.A. and D.H. set up components for, and assembled, the T. brucei RNAi libraries; S.A., L.G., N.B. and D.H. carried out RNAi screens; D.J.T., C.H.-F., M.B. and D.H. conceived the Illumina RIT-seq approach; D.J.T. carried out Illumina DNA sequencing; A.S.F., C.H.-F. and M.B. developed protocols for sequence mapping and analysis; A.S. carried out sequence mapping and analysis; S.H. developed our more recent protocols for sequence mapping and analysis; S.A., L.G. and N.B. characterized hits; L.G., S.A., D.J.T., N.B., A.S.F. and S.H. wrote the protocols; and D.H. wrote other sections of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Method 1
Additional details regarding routine handling of Trypanosoma brucei. (PDF 56 kb)
Supplementary Method 2
RITseq.py — Python script for sequence mapping. (ZIP 1 kb)
Rights and permissions
About this article
Cite this article
Glover, L., Alsford, S., Baker, N. et al. Genome-scale RNAi screens for high-throughput phenotyping in bloodstream-form African trypanosomes. Nat Protoc 10, 106–133 (2015). https://doi.org/10.1038/nprot.2015.005
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2015.005
This article is cited by
-
Spatial integration of transcription and splicing in a dedicated compartment sustains monogenic antigen expression in African trypanosomes
Nature Microbiology (2021)
-
Monoallelic expression and epigenetic inheritance sustained by a Trypanosoma brucei variant surface glycoprotein exclusion complex
Nature Communications (2019)
-
High-resolution analysis of multi-copy variant surface glycoprotein gene expression sites in African trypanosomes
BMC Genomics (2016)
-
Drug resistance in eukaryotic microorganisms
Nature Microbiology (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.