Abstract
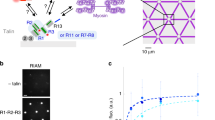
In many mechanosensitive biological processes, actin-binding proteins (ABPs) sense the force generated by the actomyosin cytoskeleton and respond by recruiting effector proteins. We developed an in vitro assay, with pure proteins, to observe the force-dependent binding of a protein to a cryptic binding site buried in the stretchable domain of an ABP. Here we describe the protocol to study the actomyosin-dependent binding of vinculin to the ABP talin. In this assay, talin is immobilized in 5-μm-diameter disc-shaped islands, which are regularly spaced by 35 μm and micropatterned on a glass coverslip. In response to the force generated by an actomyosin network, talin extension reveals cryptic vinculin-binding sites (VBSs). To follow this reaction, fluorescent proteins are visualized by total internal refection fluorescence (TIRF) microscopy. EGFP-vinculin fluorescence in talin-coated discs reveals the binding of vinculin to stretched talin. Actomyosin structures are visualized by the fluorescence of Alexa Fluor 594–labeled actin. This protocol describes the purification of the proteins, the preparation of the chamber in which talin is coated on a micropatterned surface, and the biochemical conditions to study several kinetic parameters of the actomyosin-dependent binding of vinculin to talin. A stable actomyosin network is used to measure the steady-state dissociation of vinculin from talin under constant force. In the presence of α-actinin-1, actomyosin cables undergo cycles of force application and release, allowing the measurement of vinculin dissociation associated with talin re-folding. Expression and purification of the proteins requires at least 3 weeks. The assay can be completed within 1 d.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hoffman, B.D., Grashoff, C. & Schwartz, M.A. Dynamic molecular processes mediate cellular mechanotransduction. Nature 475, 316–323 (2011).
Gardel, M.L., Schneider, I.C., Aratyn-Schaus, Y. & Waterman, C.M. Mechanical integration of actin and adhesion dynamics in cell migration. Annu. Rev. Cell Dev. Biol. 26, 315–333 (2010).
Geiger, B., Spatz, J.P. & Bershadsky, A.D. Environmental sensing through focal adhesions. Nat. Rev. Mol. Cell Biol. 10, 21–33 (2009).
Rief, M., Gautel, M., Oesterhelt, F., Fernandez, J.M. & Gaub, H.E. Reversible unfolding of individual titin immunoglobulin domains by AFM. Science 276, 1109–1112 (1997).
del Rio, A. et al. Stretching single talin rod molecules activates vinculin binding. Science 323, 638–641 (2009).
Ciobanasu, C., Faivre, B. & Le Clainche, C. Actomyosin-dependent formation of the mechanosensitive talin-vinculin complex reinforces actin anchoring. Nat. Commun. 5, 3095 (2014).
Yao, M. et al. Mechanical activation of vinculin binding to talin locks talin in an unfolded conformation. Sci. Rep. 4, 4610 (2014).
Margadant, F. et al. Mechanotransduction in vivo by repeated talin stretch-relaxation events depends upon vinculin. PLoS Biol. 9, e1001223 (2011).
Hirata, H., Tatsumi, H., Lim, C.T. & Sokabe, M. Force-dependent vinculin binding to talin in live cells: a crucial step in anchoring the actin cytoskeleton to focal adhesions. Am. J. Physiol. Cell Physiol. 306, C607–C620 (2014).
Ciobanasu, C., Faivre, B. & Le Clainche, C. Integrating actin dynamics, mechanotransduction and integrin activation: the multiple functions of actin binding proteins in focal adhesions. Eur. J. Cell Biol. 92, 339–348 (2013).
Yonemura, S., Wada, Y., Watanabe, T., Nagafuchi, A. & Shibata, M. Alpha-Catenin as a tension transducer that induces adherens junction development. Nat. Cell Biol. 12, 533–542 (2010).
Aragona, M. et al. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 154, 1047–1059 (2013).
Wirtz, D., Konstantopoulos, K. & Searson, P.C. The physics of cancer: the role of physical interactions and mechanical forces in metastasis. Nat. Rev. Cancer 11, 512–522 (2011).
Hu, K., Ji, L., Applegate, K.T., Danuser, G. & Waterman-Storer, C.M. Differential transmission of actin motion within focal adhesions. Science 315, 111–115 (2007).
Murrell, M., Thoresen, T. & Gardel, M. Reconstitution of contractile actomyosin arrays. Methods Enzymol. 540, 265–282 (2014).
Thoresen, T., Lenz, M. & Gardel, M.L. Thick filament length and isoform composition determine self-organized contractile units in actomyosin bundles. Biophys. J. 104, 655–665 (2013).
Reymann, A.C. et al. Nucleation geometry governs ordered actin networks structures. Nat. Mater. 9, 827–832 (2010).
Reymann, A.C. et al. Actin network architecture can determine myosin motor activity. Science 336, 1310–1314 (2012).
Le Clainche, C. & Carlier, M.F. Regulation of actin assembly associated with protrusion and adhesion in cell migration. Physiol. Rev. 88, 489–513 (2008).
Carlier, M.F., Le Clainche, C., Wiesner, S. & Pantaloni, D. Actin-based motility: from molecules to movement. BioEssays 25, 336–345 (2003).
Nag, S., Larsson, M., Robinson, R.C. & Burtnick, L.D. Gelsolin: the tail of a molecular gymnast. Cytoskeleton (Hoboken) 70, 360–384 (2013).
Selve, N. & Wegner, A. Rate constants and equilibrium constants for binding of the gelsolin-actin complex to the barbed ends of actin filaments in the presence and absence of calcium. Eur. J. Biochem. 160, 379–387 (1986).
Wiesner, S. et al. A biomimetic motility assay provides insight into the mechanism of actin-based motility. J. Cell Biol. 160, 387–398 (2003).
Bernheim-Groswasser, A., Wiesner, S., Golsteyn, R.M., Carlier, M.F. & Sykes, C. The dynamics of actin-based motility depend on surface parameters. Nature 417, 308–311 (2002).
Kuo, J.C., Han, X., Hsiao, C.T., Yates, J.R. III & Waterman, C.M. Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for β-Pix in negative regulation of focal adhesion maturation. Nat. Cell Biol. 13, 383–393 (2011).
Schiller, H.B. & Fassler, R. Mechanosensitivity and compositional dynamics of cell-matrix adhesions. EMBO Rep. 14, 509–519 (2013).
Smith, M.A. et al. A zyxin-mediated mechanism for actin stress fiber maintenance and repair. Dev. Cell 19, 365–376 (2010).
Turner, C.E., Glenney, J.R. Jr. & Burridge, K. Paxillin: a new vinculin-binding protein present in focal adhesions. J. Cell Biol. 111, 1059–1068 (1990).
Sawada, Y. et al. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell 127, 1015–1026 (2006).
Klotzsch, E. et al. Fibronectin forms the most extensible biological fibers displaying switchable force-exposed cryptic binding sites. Proc. Natl. Acad. Sci. USA 106, 18267–18272 (2009).
Riedl, J. et al. Lifeact: a versatile marker to visualize F-actin. Nat. Methods 5, 605–607 (2008).
Wiesner, S. Purification of skeletal muscle actin. Cell Biology: a Laboratory Handbook 3rd edn. Vol. 2, 173–175 (2006).
Pollard, T.D. Myosin purification and characterization. Methods Cell Biol. 24, 333–371 (1982).
Le Clainche, C. & Carlier, M.F. Actin-based motility assay. Curr. Protoc. Cell Biol. Chapter 12 Unit 12.7; doi:10.1002/0471143030.cb1207s24 (2004).
Humphries, J.D. et al. Vinculin controls focal adhesion formation by direct interactions with talin and actin. J. Cell Biol. 179, 1043–1057 (2007).
Carisey, A. et al. Vinculin regulates the recruitment and release of core focal adhesion proteins in a force-dependent manner. Curr. Biol. 23, 271–281 (2013).
Acknowledgements
C.L.C. is supported by the Agence Nationale pour la Recherche (ANR-09-JCJC-0111 ADERACTIN). We thank the members of the 'Cytokeleton Dynamics and Motility' team for helpful discussions.
Author information
Authors and Affiliations
Contributions
C.C. and C.L.C. developed the microscopy assay, and acquired and analyzed the data presented in this protocol. B.F. cloned the cDNA and established the protocols to purify the proteins presented in this article. C.L.C. designed the experiments, supervised the project and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Ciobanasu, C., Faivre, B. & Le Clainche, C. Reconstituting actomyosin-dependent mechanosensitive protein complexes in vitro. Nat Protoc 10, 75–89 (2015). https://doi.org/10.1038/nprot.2014.200
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2014.200
This article is cited by
-
Talin dissociates from RIAM and associates to vinculin sequentially in response to the actomyosin force
Nature Communications (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.