Abstract
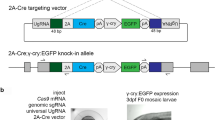
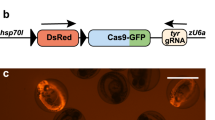
Here we present a protocol for the conversion of eGFP-transgenic zebrafish lines into lines expressing Gal4 from the same locus. This conversion allows the in-depth analysis of the former eGFP-expressing cell population; with the Gal4-upstream activating sequence (UAS) system, diverse UAS transgenes can be transactivated. Site-specific targeting of the gene encoding eGFP is achieved using the clustered regularly interspaced short palindromic repeats/CRISPR-associated 9 (CRISPR/Cas9) system. A single-guide RNA (sgRNA) that targets eGFP is injected into embryos together with a donor vector containing an optimized version of Gal4 (KalTA4) to trigger integration of the donor into the targeted eGFP genomic location. To enable screening for successful integration events, injection is performed in a UAS:RFP transgenic background; fish showing mosaic eGFP-to-RFP conversion are raised to adulthood. The progeny of these adult fish are then screened for stable germline transmission, and converted progeny are used to generate stable lines. We have been able to generate two stably converted transgenic lines within 4 months.
This is a preview of subscription content, access via your institution
Access options






Similar content being viewed by others
References
Cooper, M.S., D'Amico, L.A. & Henry, C.A. Confocal microscopic analysis of morphogenetic movements. Methods Cell Biol. 59, 179–204 (1999).
Megason, S.G. & Fraser, S.E. Digitizing life at the level of the cell: high-performance laser-scanning microscopy and image analysis for in toto imaging of development. Mech. Dev. 120, 1407–1420 (2003).
Kawakami, K. Transgenesis and gene trap methods in zebrafish by using the Tol2 transposable element. Methods Cell Biol. 77, 201–222 (2004).
Kawakami, K. et al. A transposon-mediated gene trap approach identifies developmentally regulated genes in zebrafish. Dev. Cell 7, 133–144 (2004).
Ellingsen, S. et al. Large-scale enhancer detection in the zebrafish genome. Development 132, 3799–3811 (2005).
Kawakami, K. et al. zTrap: zebrafish gene trap and enhancer trap database. BMC Dev. Biol. 10, 105 (2010).
Distel, M., Hocking, J.C., Volkmann, K. & Koster, R.W. The centrosome neither persistently leads migration nor determines the site of axonogenesis in migrating neurons in vivo. J. Cell Biol. 191, 875–890 (2010).
Wyart, C. & Del Bene, F. Let there be light: zebrafish neurobiology and the optogenetic revolution. Rev. Neurosci. 22, 121–130 (2011).
Del Bene, F. & Wyart, C. Optogenetics: a new enlightenment age for zebrafish neurobiology. Dev. Neurobiol. 72, 404–414 (2012).
Konermann, S. et al. Optical control of mammalian endogenous transcription and epigenetic states. Nature 500, 472–476 (2013).
Wojtovich, A.P. & Foster, T.H. Optogenetic control of ROS production. Redox Biol. 2, 368–376 (2014).
Airan, R.D., Thompson, K.R., Fenno, L.E., Bernstein, H. & Deisseroth, K. Temporally precise in vivo control of intracellular signalling. Nature 458, 1025–1029 (2009).
Koster, R.W. & Fraser, S.E. Tracing transgene expression in living zebrafish embryos. Dev. Biol. 233, 329–346 (2001).
Scheer, N. & Campos-Ortega, J.A. Use of the Gal4-UAS technique for targeted gene expression in the zebrafish. Mech. Dev. 80, 153–158 (1999).
Distel, M., Wullimann, M.F. & Köster, R.W. Optimized Gal4 genetics for permanent gene expression mapping in zebrafish. Proc. Natl. Acad. Sci. USA 106, 13365–13370 (2009).
Asakawa, K. et al. Genetic dissection of neural circuits by Tol2 transposon–mediated Gal4 gene and enhancer trapping in zebrafish. Proc. Natl. Acad. Sci. USA 105, 1255–1260 (2008).
Mali, P., Esvelt, K.M. & Church, G.M. Cas9 as a versatile tool for engineering biology. Nat. Methods 10, 957–963 (2013).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
Cho, S.W., Kim, S., Kim, J.M. & Kim, J.S. Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nat. Biotechnol. 31, 230–232 (2013).
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013).
Hwang, W.Y. et al. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat. Biotechnol. 31, 227–229 (2013).
Mali, P. et al. RNA-guided human genome engineering via Cas9. Science 339, 823–826 (2013).
Sander, J.D. & Joung, J.K. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat. Biotechnol. 32, 347–355 (2014).
Auer, T.O. & Del Bene, F. CRISPR/Cas9 and TALEN-mediated knock-in approaches in zebrafish. Methods 69, 142–150 (2014).
Chang, N. et al. Genome editing with RNA-guided Cas9 nuclease in zebrafish embryos. Cell Res. 23, 465–472 (2013).
Xiao, A. et al. Chromosomal deletions and inversions mediated by TALENs and CRISPR/Cas in zebrafish. Nucleic Acids Res. 41, e141 (2013).
Hruscha, A. et al. Efficient CRISPR/Cas9 genome editing with low off-target effects in zebrafish. Development 140, 4982–4987 (2013).
Auer, T.O., Duroure, K., De Cian, A., Concordet, J.P. & Del Bene, F. Highly efficient CRISPR/Cas9-mediated knock-in in zebrafish by homology-independent DNA repair. Genome Res. 24, 142–153 (2014).
Zu, Y. et al. TALEN-mediated precise genome modification by homologous recombination in zebrafish. Nat. Methods 10, 329–331 (2013).
Yoshikawa, S., Kawakami, K. & Zhao, X.C. G2R Cre reporter transgenic zebrafish. Dev. Dyn. 237, 2460–2465 (2008).
Liu, X., Li, Z., Emelyanov, A., Parinov, S. & Gong, Z. Generation of oocyte-specifically expressed Cre transgenic zebrafish for female germline excision of loxP-flanked transgene. Dev. Dyn. 237, 2955–2962 (2008).
Liu, W.Y., Wang, Y., Qin, Y., Wang, Y.P. & Zhu, Z.Y. Site-directed gene integration in transgenic zebrafish mediated by Cre recombinase using a combination of mutant lox sites. Mar. Biotechnol. (NY) 9, 420–428 (2007).
Le, X. et al. Heat shock-inducible Cre/lox approaches to induce diverse types of tumors and hyperplasia in transgenic zebrafish. Proc. Natl. Acad. Sci. USA 104, 9410–9415 (2007).
Thummel, R. et al. Cre-mediated site-specific recombination in zebrafish embryos. Dev. Dyn. 233, 1366–1377 (2005).
Langenau, D.M. et al. Cre/lox-regulated transgenic zebrafish model with conditional myc-induced T cell acute lymphoblastic leukemia. Proc. Natl. Acad. Sci. USA 102, 6068–6073 (2005).
Pan, X., Wan, H., Chia, W., Tong, Y. & Gong, Z. Demonstration of site-directed recombination in transgenic zebrafish using the Cre/loxP system. Transgenic Res. 14, 217–223 (2005).
Dong, J. & Stuart, G.W. Transgene manipulation in zebrafish by using recombinases. Methods Cell Biol. 77, 363–379 (2004).
Emelyanov, A. & Parinov, S. Mifepristone-inducible LexPR system to drive and control gene expression in transgenic zebrafish. Dev. Biol. 320, 113–121 (2008).
Esengil, H., Chang, V., Mich, J.K. & Chen, J.K. Small-molecule regulation of zebrafish gene expression. Nat. Chem. Biol. 3, 154–155 (2007).
Zhu, P. et al. Optogenetic dissection of neuronal circuits in zebrafish using viral gene transfer and the tet system. Front. Neural Circ. 3, 21 (2009).
Huang, C.J. et al. Conditional expression of a myocardium-specific transgene in zebrafish transgenic lines. Dev. Dyn. 233, 1294–1303 (2005).
Subedi, A. et al. Adoption of the Q transcriptional regulatory system for zebrafish transgenesis. Methods 66, 433–440 (2013).
Suli, A., Guler, A.D., Raible, D.W. & Kimelman, D. A targeted gene expression system using the tryptophan repressor in zebrafish shows no silencing in subsequent generations. Development 141, 1167–1174 (2014).
Abe, G., Suster, M.L. & Kawakami, K. Tol2-mediated transgenesis, gene trapping, enhancer trapping, and the Gal4-UAS system. Methods Cell Biol. 104, 23–49 (2011).
Gagnon, J.A. et al. Efficient mutagenesis by Cas9 protein-mediated oligonucleotide insertion and large-scale assessment of single-guide RNAs. PLoS ONE 9, e98186 (2014).
Hwang, W.Y. et al. Heritable and precise zebrafish genome editing using a CRISPR-Cas system. PLoS ONE 8, e68708 (2013).
Montague, T.G., Cruz, J.M., Gagnon, J.A., Church, G.M. & Valen, E. CHOPCHOP: a CRISPR/Cas9 and TALEN web tool for genome editing. Nucleic Acids Res. 42, W401–W407 (2014).
Szymczak, A.L. et al. Correction of multi-gene deficiency in vivo using a single 'self-cleaving' 2A peptide-based retroviral vector. Nat. Biotechnol. 22, 589–594 (2004).
Liu, D. et al. Efficient gene targeting in zebrafish mediated by a zebrafish-codon-optimized Cas9 and evaluation of off-targeting effect. J. Genet. Genomics 41, 43–46 (2014).
Jao, L.E., Wente, S.R. & Chen, W. Efficient multiplex biallelic zebrafish genome editing using a CRISPR nuclease system. Proc. Natl. Acad. Sci. USA 110, 13904–13909 (2013).
Balciuniene, J. & Balciunas, D. Gene trapping using Gal4 in zebrafish. J. Vis. Exp. e50113 (2013).
Westerfield, M. The Zebrafish Book 4th edn. (University of Oregon Press, 2000).
Rembold, M., Lahiri, K., Foulkes, N.S. & Wittbrodt, J. Transgenesis in fish: efficient selection of transgenic fish by co-injection with a fluorescent reporter construct. Nat. Protoc. 1, 1133–1139 (2006).
Sambrook, J. & Russell, D.W. Purification of nucleic acids by extraction with phenol:chloroform. CSH Protoc. 2006 10.1101/pdb.prot4455 (2006).
Suster, M.L., Abe, G., Schouw, A. & Kawakami, K. Transposon-mediated BAC transgenesis in zebrafish. Nat. Protoc. 6, 1998–2021 (2011).
Foley, J.E. et al. Targeted mutagenesis in zebrafish using customized zinc-finger nucleases. Nat. Protoc. 4, 1855–1867 (2009).
Meyer, M.P. & Smith, S.J. Evidence from in vivo imaging that synaptogenesis guides the growth and branching of axonal arbors by two distinct mechanisms. J. Neurosci. 26, 3604–3614 (2006).
Shin, J., Park, H.C., Topczewska, J.M., Mawdsley, D.J. & Appel, B. Neural cell fate analysis in zebrafish using olig2 BAC transgenics. Methods Cell Sci. 25, 7–14 (2003).
Ng, A.N. et al. Formation of the digestive system in zebrafish: III. Intestinal epithelium morphogenesis. Dev. Biol. 286, 114–135 (2005).
Komisarczuk, A.Z. et al. Enhancer detection and developmental expression of zebrafish sprouty1, a member of the fgf8 synexpression group. Dev. Dyn. 237, 2594–2603 (2008).
Acknowledgements
Special thanks to C. Wyart and M. Kapsimali for the Tg(olig2:eGFP), Tg(nkx2.2a:meGFP) and Tg(CLGY786) lines. We thank J. Wittbrodt for scientific discussion and support, and C. Giovannangeli and members of the Del Bene laboratory for general discussion and comments. We thank the members of the Developmental Biology Curie imaging facility (PICT-IBiSA@BDD, UMR 3215/U934) for their help and advice with confocal microscopy. Del Bene laboratory 'Neural Circuits Development' is part of the Laboratoire d'Excellence (LABEX) entitled DEEP (ANR -11-LABX-0044). T.O.A. was supported by a Boehringer Ingelheim Fonds Ph.D. fellowship. This work has been supported by an ATIP/AVENIR program starting grant (F.D.B.), by ERC-StG no. 311159 (F.D.B.), by ANR TEFOR (J.-P.C.), and by CNRS, INSERM, Institut Curie and the Muséum National d'Histoire Naturelle.
Author information
Authors and Affiliations
Contributions
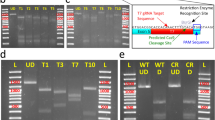
T.O.A., J.-P.C. and F.D.B. contributed to the protocol development; T.O.A. and K.D. built donor constructs, and carried out the PCR diagnosis and Southern blotting; T.O.A. performed microinjections and confocal imaging; T.O.A. and F.D.B. wrote the manuscript with inputs from J.-P.C. and K.D.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Plasmid map of the donor plasmid.
The corresponding Genbank sequence can be found in Supplementary Note 1.
Supplementary information
Rights and permissions
About this article
Cite this article
Auer, T., Duroure, K., Concordet, JP. et al. CRISPR/Cas9-mediated conversion of eGFP- into Gal4-transgenic lines in zebrafish. Nat Protoc 9, 2823–2840 (2014). https://doi.org/10.1038/nprot.2014.187
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2014.187
This article is cited by
-
Expression of a Barhl1a reporter in subsets of retinal ganglion cells and commissural neurons of the developing zebrafish brain
Scientific Reports (2020)
-
Functional analysis of the UL24 protein of suid herpesvirus 1
Virus Genes (2019)
-
Generation and characterization of UL41 null pseudorabies virus variant in vitro and in vivo
Virology Journal (2018)
-
A simplified method for identifying early CRISPR-induced indels in zebrafish embryos using High Resolution Melting analysis
BMC Genomics (2016)
-
Targeted isolation and cloning of 100-kb microbial genomic sequences by Cas9-assisted targeting of chromosome segments
Nature Protocols (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.