Abstract
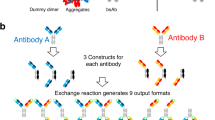
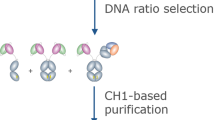
The generation of bispecific antibodies (bsAbs) with natural IgG architecture in a practical and efficient manner has been a longstanding challenge. Here we describe controlled Fab-arm exchange (cFAE), which is an easy-to-use method to generate bispecific IgG1 (bsIgG1). The protocol involves the following: (i) separate expression of two parental IgG1s containing single matching point mutations in the CH3 domain; (ii) mixing of parental IgG1s under permissive redox conditions in vitro to enable recombination of half-molecules; (iii) removal of the reductant to allow reoxidation of interchain disulfide bonds; and (iv) analysis of exchange efficiency and final product using chromatography-based or mass spectrometry (MS)–based methods. The protocol generates bsAbs with regular IgG architecture, characteristics and quality attributes both at bench scale (micrograms to milligrams) and at a mini-bioreactor scale (milligrams to grams) that is designed to model large-scale manufacturing (kilograms). Starting from good-quality purified proteins, exchange efficiencies of ≥95% can routinely be obtained within 2–3 d (including quality control).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Kontermann, R. Dual targeting strategies with bispecific antibodies. MAbs 4, 182–197 (2012).
Labrijn, A.F. et al. Species-specific determinants in the IgG CH3 domain enable Fab-arm exchange by affecting the noncovalent CH3-CH3 interaction strength. J. Immunol. 187, 3238–3246 (2011).
Schuurman, J. et al. Normal human immunoglobulin G4 is bispecific: it has two different antigen-combining sites. Immunology 97, 693–698 (1999).
van der Neut Kolfschoten, M. et al. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science 317, 1554–1557 (2007).
Gramer, M.J. et al. Production of stable bispecific IgG1 by controlled Fab-arm exchange: scalability from bench to large-scale manufacturing by application of standard approaches. MAbs 5, 962–973 (2013).
Labrijn, A.F. et al. Efficient generation of stable bispecific IgG1 by controlled Fab-arm exchange. Proc. Natl. Acad. Sci. USA 110, 5145–5150 (2013).
Kabat, E.A., Wu, T.T., Perry, H.M., Gottesman, K.S. and Foeller, C. Sequences of Proteins of Immunological Interest 5th edn. (US Department of Health and Human Services, Public Health Services, National Institutes of Health, 1991).
Birch, J.R. & Racher, A.J. Antibody production. Adv. Drug Deliv. Rev. 58, 671–685 (2006).
Demarest, S.J. & Glaser, S.M. Antibody therapeutics, antibody engineering, and the merits of protein stability. Curr. Opin. Drug Discov. Devel. 11, 675–687 (2008).
Lowe, D. et al. Aggregation, stability, and formulation of human antibody therapeutics. Adv. Protein Chem. Struct. Biol. 84, 41–61 (2011).
Chames, P. & Baty, D. Bispecific antibodies for cancer therapy. Curr. Opin. Drug Discov. Devel. 12, 276–283 (2009).
Chan, A.C. & Carter, P.J. Therapeutic antibodies for autoimmunity and inflammation. Nat. Rev. Immunol. 10, 301–316 (2010).
Wu, C. et al. Molecular construction and optimization of anti-human IL-1alpha/beta dual variable domain immunoglobulin (DVD-Ig) molecules. MAbs 1, 339–347 (2009).
Milstein, C. & Cuello, A.C. Hybrid hybridomas and their use in immunohistochemistry. Nature 305, 537–540 (1983).
Lewis, S.M. et al. Generation of bispecific IgG antibodies by structure-based design of an orthogonal Fab interface. Nat. Biotechnol. 32, 191–198 (2014).
Lindhofer, H., Mocikat, R., Steipe, B. & Thierfelder, S. Preferential species-restricted heavy/light chain pairing in rat/mouse quadromas. Implications for a single-step purification of bispecific antibodies. J. Immunol. 155, 219–225 (1995).
Merchant, A.M. et al. An efficient route to human bispecific IgG. Nat. Biotechnol. 16, 677–681 (1998).
Schaefer, W. et al. Immunoglobulin domain crossover as a generic approach for the production of bispecific IgG antibodies. Proc. Natl. Acad. Sci. USA 108, 11187–11192 (2011).
Choi, H.J., Kim, Y.J., Lee, S. & Kim, Y.S. A heterodimeric Fc-based bispecific antibody simultaneously targeting VEGFR-2 and Met exhibits potent antitumor activity. Mol. Cancer Ther. 12, 2748–2759 (2013).
Davis, J.H. et al. SEEDbodies: fusion proteins based on strand-exchange engineered domain (SEED) CH3 heterodimers in an Fc analogue platform for asymmetric binders or immunofusions and bispecific antibodies. Protein Eng. Des. Sel. 23, 195–202 (2010).
Gunasekaran, K. et al. Enhancing antibody Fc heterodimer formation through electrostatic steering effects: applications to bispecific molecules and monovalent IgG. J. Biol. Chem. 285, 19637–19646 (2010).
Moore, G.L. et al. A novel bispecific antibody format enables simultaneous bivalent and monovalent co-engagement of distinct target antigens. MAbs 3, 546–557 (2011).
Spreter Von Kreudenstein, T., Lario, P.I. & Dixit, S.B. Protein engineering and the use of molecular modeling and simulation: the case of heterodimeric Fc engineering. Methods 65, 77–94 (2014).
Strop, P. et al. Generating bispecific human IgG1 and IgG2 antibodies from any antibody pair. J. Mol. Biol. 420, 204–219 (2012).
Shatz, W. et al. Knobs-into-holes antibody production in mammalian cell lines reveals that asymmetric afucosylation is sufficient for full antibody-dependent cellular cytotoxicity. MAbs 5, 872–881 (2013).
Spiess, C. et al. Bispecific antibodies with natural architecture produced by co-culture of bacteria expressing two distinct half-antibodies. Nat. Biotechnol. 31, 753–758 (2013).
Kellner, C., Derer, S., Valerius, T. & Peipp, M. Boosting ADCC and CDC activity by Fc engineering and evaluation of antibody effector functions. Methods 65, 105–113 (2014).
Roopenian, D.C. & Akilesh, S. FcRn: the neonatal Fc receptor comes of age. Nat. Rev. Immunol. 7, 715–725 (2007).
Vink, T., Oudshoorn-Dickmann, M., Roza, M., Reitsma, J.J. & de Jong, R.N. A simple, robust and highly efficient transient expression system for producing antibodies. Methods 65, 5–10 (2014).
Bebbington, C.R. Expression of antibody genes in nonlymphoid mammalian cells. Methods 2, 136–145 (1991).
Zhao, H. et al. Formulation development of antibodies using robotic system and high-throughput laboratory (HTL). J. Pharm. Sci. 99, 2279–2294 (2010).
Acknowledgements
We thank M. Schuller for expert technical assistance and R. Hibbert, W. Vos, P. v. Berkel and M. Gramer for expert advice and helpful discussions.
Author information
Authors and Affiliations
Contributions
A.F.L., R.N.d.J., E.T.J.v.d.B., M.D.v.K., A.F.G., J.S. and P.W.H.I.P. designed the research; J.I.M., P.P., E.T.J.v.d.B. and M.D.v.K. carried out the experiments; A.F.L., J.I.M., P.P., R.N.d.J., E.T.J.v.d.B., M.D.v.K., A.F.G., J.S. and P.W.H.I.P. interpreted the data; A.F.L., J.I.M., P.P. and E.T.J.v.d.B. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
All authors are Genmab employees and have stock and/or warrants in Genmab.
Rights and permissions
About this article
Cite this article
Labrijn, A., Meesters, J., Priem, P. et al. Controlled Fab-arm exchange for the generation of stable bispecific IgG1. Nat Protoc 9, 2450–2463 (2014). https://doi.org/10.1038/nprot.2014.169
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2014.169
This article is cited by
-
Heterodimerization of T cell engaging bispecific antibodies to enhance specificity against pancreatic ductal adenocarcinoma
Journal of Hematology & Oncology (2024)
-
Subcellular trafficking and transcytosis efficacy of different receptor types for therapeutic antibody delivery at the blood‒brain barrier
Fluids and Barriers of the CNS (2023)
-
Bi- and trispecific immune cell engagers for immunotherapy of hematological malignancies
Journal of Hematology & Oncology (2023)
-
Tuning the Assembly of Bispecific Antibodies by Playing on Differential Polypeptide Chain Molar Ratios
Biotechnology and Bioprocess Engineering (2023)
-
Targeting BCMA in Multiple Myeloma
Current Hematologic Malignancy Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.