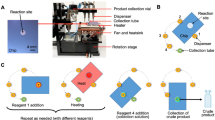
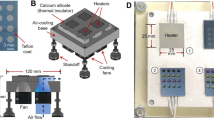
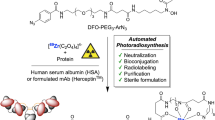
Abstract
Microfluidic techniques are increasingly being used to synthesize positron-emitting radiopharmaceuticals. Several reports demonstrate higher incorporation yields, with shorter reaction times and reduced amounts of reagents compared with traditional vessel-based techniques. Microfluidic techniques, therefore, have tremendous potential for allowing rapid and cost-effective optimization of new radiotracers. This protocol describes the implementation of a suitable microfluidic process to optimize classical 18F radiofluorination reactions by rationalizing the time and reagents used. Reaction optimization varies depending on the systems used, and it typically involves 5–10 experimental days of up to 4 h of sample collection and analysis. In particular, the protocol allows optimization of the key fluidic parameters in the first tier of experiments: reaction temperature, residence time and reagent ratio. Other parameters, such as solvent, activating agent and precursor concentration need to be stated before the experimental runs. Once the optimal set of parameters is found, repeatability and scalability are also tested in the second tier of experiments. This protocol allows the standardization of a microfluidic methodology that could be applied in any radiochemistry laboratory, in order to enable rapid and efficient radiosynthesis of new and existing [18F]-radiotracers. Here we show how this method can be applied to the radiofluorination optimization of [18F]-MEL050, a melanoma tumor imaging agent. This approach, if integrated into a good manufacturing practice (GMP) framework, could result in the reduction of materials and the time required to bring new radiotracers toward preclinical and clinical applications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Miller, P.W., Long, N.J., Vilar, R. & Gee, A.D. Synthesis of 11C, 18F, 15O, and 13N radiolabels for positron emission tomography. Angew. Chem. Int. Ed. 47, 8998–9033 (2008).
Cai, L., Lu, S. & Pike, V. Chemistry with [18F]-fluoride ion. Eur. J. Org. Chem. 2008, 2853–2873 (2008).
Pascali, G., Watts, P. & Salvadori, P.A. Microfluidics in radiopharmaceutical chemistry. Nucl. Med. Biol. 40, 776–787 (2013).
Watts, P., Pascali, G. & Salvadori, P.A. Positron emission tomography radiosynthesis in microreactors. J. Flow Chem. 2, 37–42 (2012).
Rensch, C. et al. Microfluidics: a groundbreaking technology for PET tracer production? Molecules 18, 7930–7956 (2013).
Arima, V. et al. Radiochemistry on chip: towards dose-on-demand synthesis of PET radiopharmaceuticals. Lab Chip 13, 2328–2336 (2013).
Pascali, G., Mazzone, G., Saccomanni, G., Manera, C. & Salvadori, P.A. Microfluidic approach for fast labeling optimization and dose-on-demand implementation. Nucl. Med. Biol. 37, 547–555 (2010).
Ma, X., Tseng, W.-Y., Eddings, M., Keng, P.Y. & van Dam, R.M. A microreactor with phase-change microvalves for batch chemical synthesis at high temperatures and pressures. Lab Chip 14, 280–285 (2014).
Keng, P.Y. et al. Micro-chemical synthesis of molecular probes on an electronic microfluidic device. Proc. Natl. Acad. Sci. USA 109, 690–695 (2012).
Liang, S.H. et al. Rapid microfluidic flow hydrogenation for reduction or deprotection of 18F-labeled compounds. Chem. Commun. 49, 8755–8757 (2013).
Lebedev, A. et al. Batch-reactor microfluidic device: first human use of a microfluidically produced PET radiotracer. Lab Chip 13, 136–145 (2013).
Lapi, S.E. & Welch, M.J. A historical perspective on the specific activity of radiopharmaceuticals: what have we learned in the 35 years of the ISRC? Nucl. Med. Biol. 39, 601–608 (2012).
Krasikova, R. PET radiochemistry automation: state of the art and future trends in 18F-nucleophilic fluorination. Curr. Org. Chem. 17, 2097–2107 (2013).
Scott, P.J.H., Hockley, B.G. & Kilbourn, M.R. Radiochemical Syntheses, Radiopharmaceuticals for Positron Emission Tomography Vol. 1 (John Wiley & Sons, 2012).
Pascali, G. et al. Use of non-azeotropically dried complex in microfluidic radiofluorinations. J. Nucl. Med. Meeting Abstracts 53, 578 (2012).
Kim, H.W. et al. Rapid synthesis of [18F]FDG without an evaporation step using an ionic liquid. Appl. Radiat. Isot. 61, 1241–1246 (2004).
Aerts, J. et al. Fast production of highly concentrated reactive [18F] fluoride for aliphatic and aromatic nucleophilic radiolabelling. Tetrahedron Lett. 51, 64–66 (2010).
Wessmann, S.H., Henriksen, G. & Wester, H. Cryptate mediated nucleophilic 18F-fluorination without azeotropic drying. Nuklearmedizin. 51, 1–8 (2012).
Matesic, L. et al. Ascertaining the suitability of aryl sulfonyl fluorides for [18F]radiochemistry applications: a systematic investigation using microfluidics. J. Org. Chem. 78, 11262–11270 (2013).
Lu, S., Giamis, A.M. & Pike, V.W. Synthesis of [18F]fallypride in a micro-reactor: rapid optimization and multiple-production in small doses for micro-PET studies. Curr. Radiopharm. 2, 49–55 (2009).
Pascali, G., Nannavecchia, G., Pitzianti, S. & Salvadori, P.A. Dose-on-demand of diverse 18F-fluorocholine derivatives through a two-step microfluidic approach. Nucl. Med. Biol. 38, 637–644 (2011).
Graskemper, J.W., Wang, B., Qin, L., Neumann, K.D. & DiMagno, S.G. Unprecedented directing group ability of cyclophanes in arene fluorinations with diaryliodonium salts. Org. Lett. 13, 3158–3161 (2011).
Pascali, G., Kiesewetter, D.O., Salvadori, P.A. & Eckelman, W.C. Use of 1, 8-bis-(dimethylamino)-naphthalene/H18F complex as new radiofluorinating agent. J. Labelled Comp. Radiopharm. 47, 373–383 (2004).
Greguric, I. et al. Radiosynthesis of a novel PET fluoronicotinamide for melanoma rumour PET imaging; [18F]MEL050. Aust. J. Chem. 64, 873–879 (2011).
Bouvet, V., Wuest, M., Tam, P.H., Wang, M. & Wuest, F. Microfluidic technology: an economical and versatile approach for the synthesis of O-(2-[18F]fluoroethyl)-L-tyrosine ([18F]FET). Bioorg. Med. Chem. Lett. 22, 2291–2295 (2012).
Ungersboeck, J. et al. Radiolabeling of [18F]altanserin: a microfluidic approach. Nucl. Med. Biol. 39, 1087–1092 (2012).
Dahl, K., Schou, M. & Halldin, C. Radiofluorination and reductive amination using a microfluidic device. J. Labelled Comp. Radiopharm. 55, 455–459 (2012).
Acknowledgements
We thank R. Manning and N. Paneras from Australian Nuclear Science and Technology Organisation (ANSTO) LifeSciences for the cyclotron production of [18F]-fluoride, and A. Kallinen from University of Helsinki for help in testing the protocol and data collection.
Author information
Authors and Affiliations
Contributions
G.P., L.M. and N.W. wrote the manuscript; T.L.C., B.H.F. and T.Q.P. reviewed the manuscript and tested the protocol; and P.A.S. and I.G. supervised the research.
Corresponding author
Ethics declarations
Competing interests
T.L.C. is an employee of Advion, Inc. The remaining authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Pascali, G., Matesic, L., Collier, T. et al. Optimization of nucleophilic 18F radiofluorinations using a microfluidic reaction approach. Nat Protoc 9, 2017–2029 (2014). https://doi.org/10.1038/nprot.2014.137
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2014.137
This article is cited by
-
Microliter-scale reaction arrays for economical high-throughput experimentation in radiochemistry
Scientific Reports (2022)
-
18F-labelling innovations and their potential for clinical application
Clinical and Translational Imaging (2018)
-
Synthesis and preliminary PET imaging of 11C and 18F isotopologues of the ROS1/ALK inhibitor lorlatinib
Nature Communications (2017)
-
One-step 18F labeling of biomolecules using organotrifluoroborates
Nature Protocols (2015)
-
Microprocessor-based integration of microfluidic control for the implementation of automated sensor monitoring and multithreaded optimization algorithms
Biomedical Microdevices (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.