Abstract
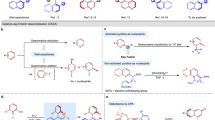
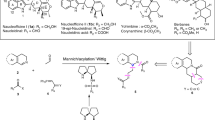
This protocol describes a method for the laboratory synthesis of enantiomerically enriched, chiral tetrahydroisoquinolines through the application of a chiral sulfinamido urea catalyst for the Povarov reaction. Tetrahydroisoquinolines are bicyclic organic frameworks present in a wide assortment of natural and synthetic biologically important compounds including martinelline, scoulerine and tubocurarine. The methodology involves the [4+2] cycloaddition of a N-arylimines with electron-rich olefins such as vinyl lactams and dihydropyrroles in the presence of a two-catalyst system consisting of an achiral strong Brønsted acid (o-nitrobenzenesulfonic acid), together with the chiral sulfinamido urea derivative 1. The anion-binding properties of the urea lead to the association of the ion pair that results from protonation of the imine substrate. Cycloaddition is followed by spontaneous proton loss with re-aromatization to provide the tetrahydroisoquinoline products in highly enantio-enriched form.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Eigen, M. Proton transfer acid-base catalysis: enzymatic hydrolysis. Part 1. Elementary processes. Angew. Chem. Int. Ed. 3, 1–19 (1964).
Akiyama, T., Itoh, J., Yokota, K. & Fuchibe, K. Enantioselective Mannich-type reaction catalyzed by a chiral Brønsted acid. Angew. Chem. Int. Ed. 43, 1566–1568 (2004).
Uraguchi, D., Sorimachi, K. & Terada, M. Organocatalytic asymmetric aza-Friedel-Crafts alkylation of furan. J. Am. Chem. Soc. 126, 11804–11805 (2004).
Nakashima, D. & Yamamoto, H. Design of chiral N-triflyl phosphoramide as a strong chiral Brønsted acid and its application to asymmetric Diels-Alder reaction. J. Am. Chem. Soc. 128, 9626–9627 (2006).
Hatano, M., Maki, T., Moriyama, K., Arinobe, M. & Ishihara, K. Pyridinium 1,1′-binaphthyl-2,2′-disulfonates as highly effective chiral Brønsted acid-base combined salt catalysts for enantioselective Mannich-type reaction. J. Am. Chem. Soc. 130, 16858–16860 (2008).
Ishihara, K., Kaneeda, M. & Yamamoto, H. Lewis-acid assisted chiral Brønsted acid for enantioselective protonation of silyl enol ethers and ketene bis(trialkyl silyl) acetals. J. Am. Chem. Soc. 116, 11179–11180 (1994).
Yamamoto, H. & Futatsugi, K. 'Designer acids': combined acid catalysis for asymmetric synthesis. Angew. Chem. Int. Ed. 44, 1924–1942 (2005).
Raheem, I.T., Thiara, P.S., Peterson, E.A. & Jacobsen, E.N. Enantioselective Pictet-Spengler-type cyclizations of hydroxylactams: H-bond donor catalysis by anion binding. J. Am. Chem. Soc. 129, 13404–13405 (2007).
Doyle, A.G. & Jacobsen, E.N. Small-molecule H-bond donors in asymmetric catalysis. Chem. Rev. 107, 5713–5743 (2007).
Schreiner, P.R. & Wittkopp, A. H-bonding additives act like Lewis acid catalysts. Org. Lett. 4, 217–220 (2002).
Kouznetsov, V.V. Recent synthetic developments in a powerful imino Diels-Alder reaction (Povarov reaction): application to the synthesis of N-polyheterocycles and related alkaloids. Tetrahedron 65, 2721–2750 (2009).
Xu, H., Zuend, S.J., Woll, M.G., Tao, Y. & Jacobsen, E.N. Asymmetric cooperative catalysis of strong Brønsted acid-promoted reactions using chiral ureas. Science 327, 986–990 (2010).
Gerard, B. et al. Application of a catalytic asymmetric Povarov reaction using chiral ureas to the synthesis of a tetrahydroquinoline library. ACS Combi. Sci. 14, 621–630 (2012).
Tan, K.L. & Jacobsen, E.N. Indium-mediated asymmetric allylation of acylhydrazones using a chiral urea catalyst. Angew. Chem. Int. Ed. 46, 1315–1317 (2007).
Ishitani, H. & Kobayashi, S. Catalytic asymmetric aza Diels-Alder reactions using a chiral lanthanide Lewis acid. Enantioselective synthesis of tetrahydroquinoline derivatives using a catalytic amount of a chiral source. Tetrahedron Lett. 37, 7357–7360 (1996).
Akiyama, T., Morita, H. & Fuchibe, K. Chiral Brønsted acid-catalyzed inverse electron-demand aza Diels-Alder reaction. J. Am. Chem. Soc. 128, 13070–13071 (2006).
Liu, H., Dagousset, G., Masson, G., Retailleau, P. & Zhu, J.P. Chiral Brønsted acid-catalyzed enantioselective three-component Povarov reaction. J. Am. Chem. Soc. 131, 4598–4599 (2009).
Witherup, K.M. et al. Martinelline and martinellic acid, novel G-protein linked receptor antagonists from the tropical plant Martinella iquitosensis (Bignoniaceae). J. Am. Chem. Soc. 117, 6682–6685 (1995).
Xia, C.F., Heng, L.S. & Ma, D.W. Total synthesis of (±)-martinelline. Tetrahedron Lett. 43, 9405–9409 (2002).
Batey, R.A. et al. A three-component coupling protocol for the synthesis of substituted hexahydropyrrolo[3,2-c]quinolines. Chem. Commun. 651–652 (1999).
Keinicke, L., Fristrup, P., Norrby, P.-O. & Madsen, R. Nonradical zinc-Barbier reaction for diastereoselective synthesis of vicinal amino alcohols. J. Am. Chem. Soc. 127, 15756–15761 (2005).
Kraus, G.A. & Neuenschwander, K. Facile synthesis of N-acyl-2-pyrrolines. J. Org. Chem. 46, 4791–4792 (1981).
Trost, B.M. & Marrs, C.M. A [3+2] cycloaddition and [4+3] cycloaddition approach to N-heterocycles via palladium-catalyzed TMM reactions with imines. J. Am. Chem. Soc. 115, 6636–6645 (1993).
Larrow, J.F. & Jacobsen, E.N. (R,R)-N,N′-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediamino manganese(III) chloride, a highly enantioselective epoxidation catalyst. Org. Syn. 75, 1 (1998).
Acknowledgements
This work was carried out with support from the US National Institutes of Health (grants no. GM-43214 and P50 GM-69721), and by fellowship support from the Dreyfus Foundation (to H.X.).
Author information
Authors and Affiliations
Contributions
H.X. designed and performed the experiments, and co-wrote the paper. H.Z. performed the synthesis of catalyst 1. E.N.J. designed and supervised the experiments, analyzed data and co-wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Xu, H., Zhang, H. & Jacobsen, E. Chiral sulfinamidourea and strong Brønsted acid–cocatalyzed enantioselective Povarov reaction to access tetrahydroquinolines. Nat Protoc 9, 1860–1866 (2014). https://doi.org/10.1038/nprot.2014.125
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2014.125
This article is cited by
-
A reaction mode of carbene-catalysed aryl aldehyde activation and induced phenol OH functionalization
Nature Communications (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.