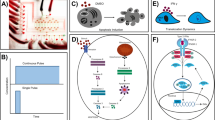
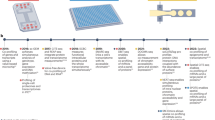
Abstract
Time-dependent analysis of dynamic processes in single live cells is a revolutionary technique for the quantitative studies of signaling networks. Here we describe an experimental pipeline and associated protocol that incorporate microfluidic cell culture, precise stimulation of cells with signaling molecules or drugs, live-cell microscopy, computerized cell tracking, on-chip staining of key proteins and subsequent retrieval of cells for high-throughput gene expression analysis using microfluidic quantitative PCR (qPCR). Compared with traditional culture dish approaches, this pipeline enhances experimental precision and throughput by orders of magnitude and introduces much-desired new capabilities in cell and fluid handling, thus representing a major step forward in dynamic single-cell analysis. A combination of microfluidic membrane valves, automation and a streamlined protocol now enables a single researcher to generate 1 million data points on single-cell protein localization within 1 week, in various cell types and densities, under 48 predesigned experimental conditions selected from different signaling molecules or drugs, their doses, timings and combinations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Fernandez-Suarez, M. & Ting, A.Y. Fluorescent probes for super-resolution imaging in living cells. Nat. Rev. Mol. Cell Biol. 9, 929–943 (2008).
Tay, S. et al. Single-cell NF-κB dynamics reveal digital activation and analogue information processing. Nature 466, 267–271 (2010).
Albeck, J.G., Mills, G.B. & Brugge, J.S. Frequency-modulated pulses of ERK activity transmit quantitative proliferation signals. Mol. Cell 49, 249–261 (2013).
You, X. et al. Intracellular protein interaction mapping with FRET hybrids. Proc. Natl. Acad. Sci. USA 103, 18458–18463 (2006).
Reits, E.A. & Neefjes, J.J. From fixed to FRAP: measuring protein mobility and activity in living cells. Nat. Cell Biol. 3, E145–147 (2001).
Kim, S.A., Heinze, K.G. & Schwille, P. Fluorescence correlation spectroscopy in living cells. Nat. Methods 4, 963–973 (2007).
Muramoto, T. et al. Live imaging of nascent RNA dynamics reveals distinct types of transcriptional pulse regulation. Proc. Natl. Acad. Sci. USA 109, 7350–7355 (2012).
Delebecque, C.J., Lindner, A.B., Silver, P.A. & Aldaye, F.A. Organization of intracellular reactions with rationally designed RNA assemblies. Science 333, 470–474 (2011).
Yunger, S., Rosenfeld, L., Garini, Y. & Shav-Tal, Y. Quantifying the transcriptional output of single alleles in single living mammalian cells. Nat. Protoc. 8, 393–408 (2013).
Puchner, E.M., Walter, J.M., Kasper, R., Huang, B. & Lim, W.A. Counting molecules in single organelles with superresolution microscopy allows tracking of the endosome maturation trajectory. Proc. Natl. Acad. Sci. USA 110, 16015–16020 (2013).
Shim, S.H. et al. Super-resolution fluorescence imaging of organelles in live cells with photoswitchable membrane probes. Proc. Natl. Acad. Sci. USA 109, 13978–13983 (2012).
Spiller, D.G., Wood, C.D., Rand, D.A. & White, M.R. Measurement of single-cell dynamics. Nature 465, 736–745 (2010).
Germain, R.N., Robey, E.A. & Cahalan, M.D. A decade of imaging cellular motility and interaction dynamics in the immune system. Science 336, 1676–1681 (2012).
Kulesa, P.M. & Fraser, S.E. Cell dynamics during somite boundary formation revealed by time-lapse analysis. Science 298, 991–995 (2002).
Cai, L., Dalal, C.K. & Elowitz, M.B. Frequency-modulated nuclear localization bursts coordinate gene regulation. Nature 455, 485–490 (2008).
Neumann, B. et al. High-throughput RNAi screening by time-lapse imaging of live human cells. Nat. Methods 3, 385–390 (2006).
Junkin, M. & Tay, S. Microfluidic single-cell analysis for systems immunology. Lab. Chip 14, 1246–1260 (2014).
Levskaya, A., Weiner, O.D., Lim, W.A. & Voigt, C.A. Spatiotemporal control of cell signalling using a light-switchable protein interaction. Nature 461, 997–1001 (2009).
Bugaj, L.J., Choksi, A.T., Mesuda, C.K., Kane, R.S. & Schaffer, D.V. Optogenetic protein clustering and signaling activation in mammalian cells. Nat. Methods 10, 249–252 (2013).
Grier, D.G. A revolution in optical manipulation. Nature 424, 810–816 (2003).
Milias-Argeitis, A. et al. In silico feedback for in vivo regulation of a gene expression circuit. Nat. Biotechnol. 29, 1114–1116 (2011).
Gómez-Sjöberg, R., Leyrat, A.A., Pirone, D.M., Chen, C.S. & Quake, S.R. Versatile, fully automated, microfluidic cell culture system. Anal. Chem. 79, 8557–8563 (2007).
Vedel, S., Tay, S., Johnston, D.M., Bruus, H. & Quake, S.R. Migration of cells in a social context. Proc. Natl. Acad. Sci. USA 110, 129–134 (2013).
Sanchez-Freire, V. et al. Microfluidic single-cell real-time PCR for comparative analysis of gene expression patterns. Nat. Protoc. 7, 829–838 (2012).
Cheong, R., Wang, C.J. & Levchenko, A. High-content cell screening in a microfluidic device. Mol. Cell. Proteom. 8, 433–442 (2009).
Cheong, R., Wang, C.J. & Levchenko, A. Using a microfluidic device for high-content analysis of cell signaling. Sci. Signal. 2, pl2 (2009).
Cheong, R., Rhee, A., Wang, C.J., Nemenman, I. & Levchenko, A. Information transduction capacity of noisy biochemical signaling networks. Science 334, 354–358 (2011).
Frank, T. & Tay, S. Flow-switching allows independently programmable, extremely stable, high-throughput diffusion-based gradients. Lab. Chip 13, 1273–1281 (2013).
Hung, P.J., Lee, P.J., Sabounchi, P., Lin, R. & Lee, L.P. Continuous perfusion microfluidic cell culture array for high-throughput cell-based assays. Biotechnol. Bioeng. 89, 1–8 (2005).
Chung, K., Rivet, C.A., Kemp, M.L. & Lu, H. Imaging single-cell signaling dynamics with a deterministic high-density single-cell trap array. Anal. Chem. 83, 7044–7052 (2011).
Faley, S.L. et al. Microfluidic single cell arrays to interrogate signalling dynamics of individual, patient-derived hematopoietic stem cells. Lab. Chip 9, 2659–2664 (2009).
Roach, K.L. et al. High-throughput single-cell bioinformatics. Biotechnol. Prog. 25, 1772–1779 (2009).
Melin, J. & Quake, S.R. Microfluidic large-scale integration: the evolution of design rules for biological automation. Ann. Rev. Biophys. Biomol. Str. 36, 213–231 (2006).
Vollmers, C., Sit, R.V., Weinstein, J.A., Dekker, C.L. & Quake, S.R. Genetic measurement of memory B-cell recall using antibody repertoire sequencing. Proc. Natl. Acad. Sci. USA 110, 13463–13468 (2013).
Kalisky, T. & Quake, S.R. Single-cell genomics. Nat. Methods 8, 311–314 (2011).
Ottesen, E.A., Hong, J.W., Quake, S.R. & Leadbetter, J.R. Microfluidic digital PCR enables multigene analysis of individual environmental bacteria. Science 314, 1464–1467 (2006).
Warren, L., Bryder, D., Weissman, I.L. & Quake, S.R. Transcription factor profiling in individual hematopoietic progenitors by digital RT-PCR. Proc. Natl. Acad. Sci. USA 103, 17807–17812 (2006).
Qin, D., Xia, Y. & Whitesides, G.M. Soft lithography for micro- and nanoscale patterning. Nat. Protoc. 5, 491–502 (2010).
Lecault, V. et al. High-throughput analysis of single hematopoietic stem cell proliferation in microfluidic cell culture arrays. Nat. Methods 8, 581–586 (2011).
Unger, M.A., Chou, H.P., Thorsen, T., Scherer, A. & Quake, S.R. Monolithic microfabricated valves and pumps by multilayer soft lithography. Science 288, 113–116 (2000).
Thorsen, T. Microfluidic large-scale integration. Science 298, 580–584 (2002).
Berthier, E., Young, E.W. & Beebe, D. Engineers are from PDMS-land, biologists are from Polystyrenia. Lab. Chip 12, 1224–1237 (2012).
Millet, L.J., Stewart, M.E., Sweedler, J.V., Nuzzo, R.G. & Gillette, M.U. Microfluidic devices for culturing primary mammalian neurons at low densities. Lab. Chip 7, 987–994 (2007).
Kolnik, M., Tsimring, L.S. & Hasty, J. Vacuum-assisted cell loading enables shear-free mammalian microfluidic culture. Lab. Chip 12, 4732–4737 (2012).
Landenberger, B., Hofemann, H., Wadle, S. & Rohrbach, A. Microfluidic sorting of arbitrary cells with dynamic optical tweezers. Lab. Chip 12, 3177–3183 (2012).
Wall, E.A. et al. Suppression of LPS-induced TNF-α production in macrophages by cAMP is mediated by PKA-AKAP95-p105. Sci. Signal 2, ra28 (2009).
Acknowledgements
We acknowledge S. Quake for supervising earlier stages of this work. We thank M. Covert and his group for useful discussions and for making an earlier version of the image analysis software available, as well as for the gift of 3T3 cells with the p65-DsRed fusion protein. This work was supported by a European Research Council (ERC) starting grant and a Swiss National Science Foundation grant to S. Tay.
Author information
Authors and Affiliations
Contributions
R.A.K. optimized the protocol and developed methods for cell retrieval and gene expression analysis, and wrote the manuscript. R.G-.S. and A.A.L. are the original developers of the microfluidic cell culture chip and software. S.T. optimized the protocol for cell signaling studies and supervised the signaling project. All authors edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
A.A.L. is an employee of Fluidigm Corporation. The remaining authors declare no competing financial interests.
Supplementary information
Supplementary Data
AutoCad DXF chip design file for cell culture chip molds (flow and control layers) (TXT 25643 kb)
Rights and permissions
About this article
Cite this article
Kellogg, R., Gómez-Sjöberg, R., Leyrat, A. et al. High-throughput microfluidic single-cell analysis pipeline for studies of signaling dynamics. Nat Protoc 9, 1713–1726 (2014). https://doi.org/10.1038/nprot.2014.120
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2014.120
This article is cited by
-
Open-source personal pipetting robots with live-cell incubation and microscopy compatibility
Nature Communications (2022)
-
A design and optimization of a high throughput valve based microfluidic device for single cell compartmentalization and analysis
Scientific Reports (2021)
-
Microfluidic reactors for advancing the MS analysis of fast biological responses
Microsystems & Nanoengineering (2019)
-
Laser-fabricated cell patterning stencil for single cell analysis
BMC Biotechnology (2017)
-
Challenges in long-term imaging and quantification of single-cell dynamics
Nature Biotechnology (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.