Abstract
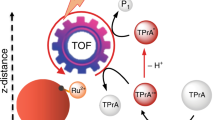
Assays using probes labeled with electrochemiluminescent moieties are extremely powerful analytical tools that are used in fields such as medical diagnostics, environmental analysis and food safety monitoring, in which sensitive, reliable and reproducible detection of biomolecules is a requirement. The most efficient electrochemiluminescence (ECL) reaction to date is based on tris(2,2′-bipyridyl)ruthenium(II) (Ru(bpy)32+) with tripropylamine (TPrA) as the co-reactant. Here we present a detailed protocol for preparing Ru(bpy)32+ probes and their bioanalytical applications. This protocol includes (i) the synthesis of a biologically active Ru(bpy)32+-N-hydroxysuccinimide (NHS) ester, (ii) its covalent labeling with both antibodies and DNA probes and (iii) the detection and quantification of ECL in a microfluidic system with a paramagnetic microbead solid support. In our magnetic bead–based ECL system, two probes are required: a capture probe (labeled with biotin to be captured by a streptavidin-coated magnetic bead) and a detector probe (labeled with Ru(bpy)32+). The complex consisting of the analyte, the capture probe, the detector probe and the magnetic bead is brought into contact with the electrode by using a magnetic field. The Ru(bpy)32+ reacts with TPrA in solution to generate the ECL signal. The full protocol, including the synthesis and labeling of the bioactive Ru(bpy)32+, requires 5–6 d to complete. ECL immunoassays or nucleic acid tests only require 1.5–2 h, including the sample preparation time.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Richter, M.M. Electrochemiluminescence (ECL). Chem. Rev. 104, 3003–3036 (2004).
Miao, W.J. Electrogenerated chemiluminescence and its biorelated applications. Chem. Rev. 108, 2506–2553 (2008).
Ma, D.L. et al. Label-free luminescent oligonucleotide-based probes. Chem. Soc. Rev. 42, 3427–3440 (2013).
Dodeigne, C., Thunus, L. & Lejeune, R. Chemiluminescence as diagnostic tool. A review. Talanta 51, 415–439 (2000).
Leung, K.H. et al. Detection of base excision repair enzyme activity using a luminescent G-quadruplex selective switch-on probe. Chem. Commun. 49, 5630–5632 (2013).
Pfleger, K.D. & Eidne, K.A. Illuminating insights into protein-protein interactions using bioluminescence resonance energy transfer (BRET). Nat. Methods 3, 165–174 (2006).
Leung, C.H. et al. Luminescent detection of DNA-binding proteins. Nucleic Acids Res. 40, 941–955 (2012).
Bard, A.J. & Faulkner, L.R. Electrochem. Methods (Wiley, 1980).
Bard, A.J. (ed.) Electrogenerated chemiluminescence (Marcel Dekker, 2004).
Knight, A.W. Electrogenerated chemiluminescence. in Chemiluminescence in Analytical Chemistry 211–247 (Marcel Dekker, 2001).
Chang, M.M., Saji, T. & Bard, A.J. Electrogenerated chemiluminescence. 30. Electrochemical oxidation of oxalate ion in the presence of luminescers in acetonitrile solutions. J. Am. Chem. Soc. 99, 5399–5403 (1977).
Rubinstein, I. & Bard, A.J. Electrogenerated chemiluminescence. 37. Aqueous ECL systems based on tris(2,2′-bipyridine)ruthenium2+ and oxalate or organic acids. J. Am. Chem. Soc. 103, 512–516 (1981).
Leland, J.K. & Powell, M.J. Electrogenerated chemiluminescence: An oxidative-reduction type ECL reaction sequence using tripropylamine. J. Electrochem. Soc. 137, 3127–3131 (1990).
Miao, W., Choi, J.-P. & Bard, A.J. Electrogenerated chemiluminescence 69: the tris(2,2′-bipyridine)ruthenium(II), (Ru(bpy)3 2+)/tri-n-propylamine (TPrA) system revisited - A new route involving TPrA•+ cation radicals. J. Am. Chem. Soc. 124, 14478–14485 (2002).
Ge, L. et al. Three-dimensional paper-based electrochemiluminescence immunodevice for multiplexed measurement of biomarkers and point-of-care testing. Biomaterials 33, 1024–1031 (2012).
Wang, X. et al. A solid-state electrochemiluminescence biosensing switch for detection of thrombin based on ferrocene-labeled molecular beacon aptamer. Biosens. Bioelectron. 24, 3288–3292 (2009).
Yuan, T., Liu, Z., Hu, L., Zhang, L. & Xu, G. Label-free supersandwich electrochemiluminescence assay for detection of sub-nanomolar Hg2+. Chem. Commun. 47, 11951–11953 (2011).
Lin, Z., Chen, L., Zhu, X., Qiu, B. & Chen, G. Signal-on electrochemiluminescence biosensor for thrombin based on target-induced conjunction of split aptamer fragments. Chem. Commun. 46, 5563–5565 (2010).
Sun, B. et al. Double covalent coupling method for the fabrication of highly sensitive and reusable electrogenerated chemiluminescence sensors. Anal. Chem. 82, 5046–5052 (2010).
Tang, X. et al. Quenching of the electrochemiluminescence of tris(2,2′-bipyridine)ruthenium (II)/tri-n-propylamine by pristine carbon nanotube and its application to quantitative detection of DNA. Anal. Chem. 85, 1711–1718 (2013).
Wu, Y., Shi, H., Yuan, L. & Liu, S. A novel electrochemiluminescence immunosensor via polymerization-assisted amplification. Chem. Commun. 46, 7763–7765 (2010).
Xu, L., Li, Y., Wu, S., Liu, X. & Su, B. Imaging latent fingerprints by electrochemiluminescence. Angew. Chem. Int. Ed. 124, 8192–8196 (2012).
Xu, X.H. & Bard, A.J. Electrogenerated chemiluminescence. 55. emission from adsorbed Ru(bpy)3 2+ on graphite, platinum, and gold. Langmuir 10, 2409–2414 (1994).
Blackburn, G.F. et al. Electrochemiluminescence detection for development of immunoassays and DNA probe assays for clinical diagnostics. Clin. Chem. 37, 91534–91539 (1991).
Yan, G.H., Xing, D., Tan, S.C. & Chen, Q. Rapid and sensitive immunomagnetic-electrochemiluminescent detection of p53 antibodies in human serum. J. Immunol. Methods 288, 47–54 (2004).
Kenten, J.H. et al. Rapid electrochemiluminescence assays of polymerase chain reaction products. Clin. Chem. 37, 1626–1632 (1991).
Zhu, D.B., Xing, D., Shen, X.Y. & Liu, J.F. A method to quantitatively detect H-ras point mutation based on electrochemiluminescence. Biochem. Biophys. Res. Commun. 324, 964–969 (2004).
Yin, X.B., Dong, S. & Wang, E. Analytical applications of the electrochemiluminescence of tris(2,2′-bipyridyl)ruthenium and its derivatives. Trends Anal. Chem. 23, 432–441 (2004).
Forster, R.J., Bertoncello, P. & Keyes, T.E. Electrogenerated chemiluminescence. Annu. Rev. Anal. Chem. 2, 359–385 (2009).
Lee, W.-Y. Tris(2,2′-bipyridyl)ruthenium(II) electrogenerated chemiluminescence in analytical science. Microchimica. Acta 127, 19–39 (1997).
Wei, H. & Wang, E.K. Solid-state electrochemiluminescence of tris(2,2′-bipyridyl) ruthenium. Trends Anal. Chem. 27, 447–459 (2008).
Hu, L.Z. & Xu, G.B. Applications and trends in electrochemiluminescence. Chem. Soc. Rev. 39, 3275–3304 (2010).
Bertoncello, P. & Forster, R.J. Nanostructured materials for electrochemiluminescence (ECL)-based detection methods: recent advances and future perspectives. Biosens. Bioelectron. 24, 3191–3200 (2009).
Kijek, T.M., Rossi, C.A., Moss, D., Parker, R.W. & Henchal, E.A. Rapid and sensitive immunomagnetic-electrochemiluminescent detection of staphyloccocal enterotoxin B. J. Immunol. Methods 236, 9–17 (2000).
Gemen, B.V. et al. A one-tube quantitative HIV-1 RNA NASBA nucleic acid amplification assay using electrochemiluminescent (ECL) labelled probes. J. Virol. Methods 49, 157–167 (1994).
Zhou, X.M., Xing, D., Zhu, D.B. & Jia, L. Magnetic bead and nanoparticle based electrochemiluminescence amplification assay for direct and sensitive measuring of telomerase activity. Anal. Chem. 81, 255–261 (2009).
Boom, R. et al. A highly sensitive assay for detection and quantitation of human cytomegalovirus DNA in serum and plasma by PCR and electrochemiluminescence. J. Clin. Microbiol. 37, 1489–1497 (1999).
Zhang, Y. et al. Magnetic beads-based electrochemiluminescence immunosensor for determination of cancer markers using quantum dot functionalized PtRu alloys as labels. Analyst 137, 2176–2182 (2012).
Zhu, X., Zhou, X.M. & Xing, D. Nano-magnetic primer based electrochemiluminescence-polymerase chain reaction (NMPE-PCR) assay. Biosens. Bioelectron. 31, 463–468 (2012).
Duan, R.X., Zhou, X.M. & Xing, D. Electrochemiluminescence biobarcode method based on cysteamine-gold nanoparticle conjugates. Anal. Chem. 82, 3099–3103 (2010).
Guglielmo-Viret, V. & Thullier, P. Comparison of an electrochemiluminescence assay in plate format over a colorimetric ELISA, for the detection of ricin B chain (RCA-B). J. Immunol. Methods 328, 70–78 (2007).
Guglielmo-Viret, V., Attrée, O., Blanco-Gros, V. & Thullier, P. Comparison of electrochemiluminescence assay and ELISA for the detection of Clostridium botulinum type B neurotoxin,. J. Immunol. Methods 301, 164–172 (2005).
Bruno, J.G. & Kiel, J.L. In vitro selection of DNA aptamers to anthrax spores with electrochemiluminescence detection. Biosens. Bioelectron. 14, 457–464 (1999).
Zhu, D.B., Tang, Y.B., Xing, D. & Chen, W.R. PCR-free quantitative detection of genetically modified organism from raw materials. An electrochemiluminescence-based bio bar code method. Anal. Chem. 80, 3566–3571 (2008).
Wu, S.L. et al. Detection of dengue viral RNA using a nucleic acid sequence-based amplification assay. J. Clin. Microbiol. 39, 2794–2798 (2001).
Antony, S. et al. Novel high-throughput electrochemiluminescent assay for identification of human tyrosyl-DNA phosphodiesterase (Tdp1) inhibitors and characterization of furamidine (NSC 305831) as an inhibitor of Tdp1. Nucleic Acids Res. 35, 4474–4484 (2007).
Li, Q., Zhou, X.M. & Xing, D. Rapid and highly sensitive detection of mercury ion (Hg2+) by magnetic beads-based electrochemiluminescence assay. Biosens. Bioelectron. 26, 859–862 (2010).
Yu, H., Raymonda, J.W., McMahon, T.M. & Campagnari, A.A. Detection of biological threat agents by immunomagnetic microsphere-based solid-phase fluorogenic- and electro-chemiluminescence. Biosens. Bioelectron. 14, 829–840 (2000).
Deiss, F. et al. Multiplexed sandwich immunoassays using electrochemiluminescence imaging resolved at the single bead level. J. Am. Chem. Soc. 131, 6088–6089 (2009).
Liu, X., Shi, L., Niu, W., Li, H. & Xu, G. Environmentally friendly and highly sensitive ruthenium(II)tris(2,2′-bipyridyl) electrochemiluminescent system using 2-(dibutylamino) ethanol as co-reactant. Angew. Chem. Int. Ed. 119, 425–428 (2007).
Cao, W., Ferrance, J.P., Demas, J. & Landers, J.P. Quenching of the electrochemiluminescence of tris(2,2′-bipyridine)ruthenium(II) by ferrocene and its potential application to quantitative DNA detection. J. Am. Chem. Soc. 128, 7572–7578 (2006).
Zhou, M., Roovers, J., Robertson, G.P. & Grover, C.P. Multilabeling biomolecules at a single site. 1. Synthesis and characterization of a dendritic label for electrochemiluminescence assays. Anal. Chem. 75, 6708–6717 (2003).
Terpetschnig, E., Szmacinski, H., Malak, H. & Lakowicz, J.R. Metal-ligand complexes as a new class of long-lived fluorophores for protein hydrodynamics. Biophys. J. 68, 342–350 (1995).
Terpetschnig, E., Szmacinski, H. & Lakowicz, J.R. Fluorescence polarization immunoassay of a high-molecular-weight antigen based on a long-lifetime Ru-ligand complex. Anal. Biochem. 227, 140–147 (1995).
Gudibande, S.R., Kenten, J.H., Link, J., Friedman, K. & Massey, R.J. Rapid, non-separation electrochemiluminescent DNA hybridization assays for PCR products, using 3′-labelled oligonucleotide probes. Mol. Cell. Probes 6, 495–503 (1992).
Su, Q., Xing, D. & Zhou, X.M. Magnetic beads-based rolling-circle amplification–electrochemiluminescence assay for highly sensitive detection of point mutation. Biosens. Bioelectron. 25, 1615–1621 (2010).
Workman, S. & Richter, M.M. The effects of nonionic surfactants on the tris(2,2′-bipyridyl)ruthenium(II)-tripropylamine electrochemiluminescence system. Anal. Chem. 72, 5556–5561 (2000).
Zu, Y. & Bard, A.J. Electrogenerated chemiluminescence. 67. dependence of light emission of the tris(2,2′)bipyridylruthenium(II)/tripropylamine system on electrode surface hydrophobicity. Anal. Chem. 73, 3960–3964 (2001).
Komori, K., Takada, K., Hatozaki, O. & Oyama, N. Electrochemiluminescence of Ru(II) complexes immobilized on a magnetic microbead surface: distribution of magnetic microbeads on the electrode surface and effect of azideion. Langmuir 23, 6446–6452 (2007).
Wang, J. et al. Amplified voltammetric detection of DNA hybridization via oxidation of ferrocene caps on gold nanoparticle/streptavidin conjugates. Anal. Chem. 75, 3941–3945 (2003).
Acknowledgements
This research is supported by the National Basic Research Program of China (2010CB732602), the National Natural Science Foundation of China (NSFC) (81101121), the Key Program of the NSFC-Guangdong Joint Funds of China (U0931005) and the Program of the Pearl River Young Talents of Science and Technology in Guangzhou, China (2013J2200021).
Author information
Authors and Affiliations
Contributions
X.Z. and D.X. conceived and designed the study, supervised the work and wrote the manuscript. X.Z., Y.L., Z.M., D.Z. and W.L. conducted the experiments and the data analysis. X.Z., W.L. and H.L. designed and prepared all figures.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 3 Characterization of the labeling of DNA by LC/MS.
LC/MS chromatogram of (A) DNA probes not labelled with Ru(bpy)32+-NHS, and (B) DNA probes labeled with Ru(bpy)32+-NHS. The mass of the unlabeled DNA probes was 6872.4, and the mass of the Ru(bpy)32+-labeled DNA probes was 7510.7. A mass increase of 638.3 after labeling indicated only a single probe labeled each DNA molecule. (Note that another NHS group unanticipated in the labeling reaction would hydrolyze to a carboxyl group).
Supplementary information
Supplementary Figure 1
ESI-MS of [Ru(bpy)2(dcbpy)(PF6)2]. (PDF 67 kb)
Supplementary Figure 2
1H NMR of [Ru(bpy)2(dcbpy)(PF6)2] in DMSO at 400 MHz. (PDF 166 kb)
Supplementary Figure 3
Characterization of the labeling of DNA by LC/MS. (PDF 167 kb)
Rights and permissions
About this article
Cite this article
Zhou, X., Zhu, D., Liao, Y. et al. Synthesis, labeling and bioanalytical applications of a tris(2,2′-bipyridyl)ruthenium(II)-based electrochemiluminescence probe. Nat Protoc 9, 1146–1159 (2014). https://doi.org/10.1038/nprot.2014.060
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2014.060
This article is cited by
-
Tris(2,2’-bipyridyl)ruthenium (II) complex as a universal reagent for the fabrication of heterogeneous electrochemiluminescence platforms and its recent analytical applications
Analytical and Bioanalytical Chemistry (2023)
-
Electrochemiluminescence biosensing and bioimaging with nanomaterials as emitters
Science China Chemistry (2022)
-
Recent advances in the potential applications of luminescence-based, SPR-based, and carbon-based biosensors
Applied Microbiology and Biotechnology (2022)
-
Spooling electrochemiluminescence spectroscopy: development, applications and beyond
Nature Protocols (2021)
-
Electrochemiluminescent immunoassay for the determination of CA15-3 and CA72-4 using graphene oxide nanocomposite modified with CdSe quantum dots and Ru(bpy)3 complex
Microchimica Acta (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.