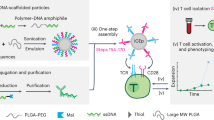
Abstract
Cell therapies enable unprecedented treatment options to replace tissues, destroy tumors and facilitate regeneration. The greatest challenge facing cell therapy is the inability to control the fate and function of cells after transplantation. We have developed an approach to control cell phenotype in vitro and after transplantation by engineering cells with intracellular depots that continuously release phenotype-altering agents for days to weeks. The platform enables control of cells' secretome, viability, proliferation and differentiation, and the platform can be used to deliver drugs or other factors (e.g., dexamethasone, rhodamine and iron oxide) to the cell's microenvironment. The preparation, efficient internalization and intracellular stabilization of ∼1-μm drug-loaded microparticles are critical for establishing sustained control of cell phenotype. Herein we provide a protocol to generate and characterize micrometer-sized agent-doped poly(lactic-co-glycolic) acid (PLGA) particles by using a single-emulsion evaporation technique (7 h), to uniformly engineer cultured cells (15 h), to confirm particle internalization and to troubleshoot commonly experienced obstacles.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
von Bahr, L. et al. Long-term complications, immunologic effects, and role of passage for outcome in mesenchymal stromal cell therapy. Biol. Blood Marrow Transplant 18, 557–564 (2012).
François, M., Romieu-Mourez, R., Li, M. & Galipeau, J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol. Ther. 20, 187–195 (2012).
Cahan, P. & Daley, G.Q. Origins and implications of pluripotent stem cell variability and heterogeneity. Nat. Rev. Mol. Cell Biol. 14, 357–368 (2013).
Fomina-Yadlin, D. et al. Small-molecule inducers of insulin expression in pancreatic alpha-cells. Proc. Natl. Acad. Sci. USA 107, 15099–15104 (2010).
Da Silva Meirelles, L. & Chagastelles, P. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 119 (Part 11): 2204–2213 (2006).
Zhukareva, V., Obrocka, M., Houle, J.D., Fischer, I. & Neuhuber, B. Secretion profile of human bone marrow stromal cells: donor variability and response to inflammatory stimuli. Cytokine 50, 317–321 (2010).
Wu, W., Ye, Z., Zhou, Y. & Tan, W.-S. AICAR, a small chemical molecule, primes osteogenic differentiation of adult mesenchymal stem cells. Int. J. Artif. Organs 34, 1128–1136 (2011).
Suzuki, Y., Kim, H.W., Ashraf, M. & Haider, H.K. Diazoxide potentiates mesenchymal stem cell survival via NF-κB–dependent miR-146a expression by targeting Fas. Am. J. Physiol. Heart Circ. Physiol. 299, H1077–82 (2010).
Dong, Q., Yang, Y., Song, L., Qian, H. & Xu, Z. Atorvastatin prevents mesenchymal stem cells from hypoxia and serum-free injury through activating AMP-activated protein kinase. Int. J. Cardiol. 153, 311–316 (2011).
Brey, D.M. et al. High-throughput screening of a small molecule library for promoters and inhibitors of mesenchymal stem cell osteogenic differentiation. Biotechnol. Bioeng. 108, 163–174 (2011).
Sarkar, D., Ankrum, J., Teo, G.S.L., Carman, C.V. & Karp, J.M. Cellular and extracellular programming of cell fate through engineered intracrine-, paracrine-, and endocrine-like mechanisms. Biomaterials 32, 3053–3061 (2011).
Slowing, I.I. et al. Exocytosis of mesoporous silica nanoparticles from mammalian cells: from asymmetric cell-to-cell transfer to protein harvesting. Small 7, 1526–1532 (2011).
Jiang, X. et al. Endo- and exocytosis of zwitterionic quantum dot nanoparticles by live HeLa cells. ACS Nano 4, 6787–6797 (2010).
Panyam, J. & Labhasetwar, V. Dynamics of endocytosis and exocytosis of poly(D,L-lactide-co-glycolide) nanoparticles in vascular smooth muscle cells. Pharm. Res. 20, 212–220 (2003).
Jin, H., Heller, D.A., Sharma, R. & Strano, M.S. Size-dependent cellular uptake and expulsion of single-walled carbon nanotubes: single particle tracking and a generic uptake model for nanoparticles. ACS Nano 3, 149–158 (2009).
Chithrani, B.D. & Chan, W.C.W. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett. 7, 1542–1550 (2007).
Swiston, A., Gilbert, J., Irvine, D., Cohen, R. & Rubner, M. Freely suspended cellular 'backpacks' lead to cell aggregate self-assembly. Biomacromolecules 4920–4925 (2010).
Stephan, M.T., Moon, J.J., Um, S.H., Bershteyn, A. & Irvine, D.J. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat. Med. 16, 1035–1041 (2010).
Xu, C. et al. Tracking mesenchymal stem cells with iron oxide nanoparticle loaded poly(lactide-co-glycolide) microparticles. Nano Lett. 12, 4131–4139 (2012).
Cohen-Sela, E., Chorny, M., Koroukhov, N., Danenberg, H.D. & Golomb, G. A new double emulsion solvent diffusion technique for encapsulating hydrophilic molecules in PLGA nanoparticles. J. Control Release 133, 90–95 (2009).
Karnik, R. et al. Microfluidic platform for controlled synthesis of polymeric nanoparticles. Nano Lett. 8, 2906–2912 (2008).
Thomas, T.T., Kohane, D.S., Wang, A. & Langer, R. Microparticulate formulations for the controlled release of interleukin-2. J. Pharm. Sci. 93, 1100–1109 (2004).
Sawada, M., Hayes, P. & Matsuyama, S. Cytoprotective membrane-permeable peptides designed from the Bax-binding domain of Ku70. Nat. Cell. Biol. 5, 352–357 (2003).
Ieda, M. et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 142, 375–386 (2010).
Murry, C.E. & Keller, G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell 132, 661–680 (2008).
Brenner, S. et al. CXCR4-transgene expression significantly improves marrow engraftment of cultured hematopoietic stem cells. Stem Cell 22, 1128–1133 (2004).
Haider, H.K., Jiang, S., Idris, N.M. & Ashraf, M. IGF-1-overexpressing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of SDF-1α/CXCR4 signaling to promote myocardial repair. Circ. Res. 103, 1300–1308 (2008).
Panyam, J. & Labhasetwar, V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv. Drug Deliv. Rev. 55, 329–347 (2003).
Panyam, J., Zhou, W.-Z., Prabha, S., Sahoo, S.K. & Labhasetwar, V. Rapid endo-lysosomal escape of poly(DL-lactide-co-glycolide) nanoparticles: implications for drug and gene delivery. FASEB J. 16, 1217–1226 (2002).
Patil, Y., Sadhukha, T., Ma, L. & Panyam, J. Nanoparticle-mediated simultaneous and targeted delivery of paclitaxel and tariquidar overcomes tumor drug resistance. J. Control Release 136, 21–29 (2009).
Acharya, S. & Sahoo, S.K. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv. Drug Deliv. Rev. 63, 170–183 (2011).
Timko, B.P. et al. Advances in drug delivery. Annu. Rev. Mater. Res. 41, 1–20 (2011).
Kostanski, J.W. & DeLuca, P.P. A novel in vitro release technique for peptide containing biodegradable microspheres. AAPS Pharm. Sci. Tech. 1, E4 (2000).
D'Souza, S.S. & DeLuca, P.P. Methods to assess in vitro drug release from injectable polymeric particulate systems. Pharm. Res. 23, 460–474 (2006).
Dokoumetzidis, A. & Macheras, P. A century of dissolution research: from Noyes and Whitney to the biopharmaceutics classification system. Int. J. Pharm. 321, 1–11 (2006).
Acknowledgements
This work was supported by US National Institutes of Health grant no. HL095722 to J.M.K. and by a Movember-PCF Challenge Award to J.M.K. J.A.A. was supported by the Hugh Hampton Young Memorial Fellowship.
Author information
Authors and Affiliations
Contributions
J.A.A., C.X., D.S., and J.M.K. conceived of the protocol and its initial applications. J.A.A., O.R.M. and K.S.N. optimized the protocol. J.A.A., O.R.M., K.S.N. and J.M.K. wrote the manuscript. J.A.A. designed and prepared all figures.
Corresponding author
Ethics declarations
Competing interests
J.M.K. is a paid consultant of Sanofi and Stempeutics in the field of mesenchymal stem cell therapy.
Rights and permissions
About this article
Cite this article
Ankrum, J., Miranda, O., Ng, K. et al. Engineering cells with intracellular agent–loaded microparticles to control cell phenotype. Nat Protoc 9, 233–245 (2014). https://doi.org/10.1038/nprot.2014.002
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2014.002
This article is cited by
-
Strategies to capitalize on cell spheroid therapeutic potential for tissue repair and disease modeling
npj Regenerative Medicine (2022)
-
Advances in Pluripotent Stem Cells: History, Mechanisms, Technologies, and Applications
Stem Cell Reviews and Reports (2020)
-
Fluorescence-based tracing of transplanted intestinal epithelial cells using confocal laser endomicroscopy
Stem Cell Research & Therapy (2019)
-
Remote targeted implantation of sound-sensitive biodegradable multi-cavity microparticles with focused ultrasound
Scientific Reports (2019)
-
Preparation and characterization of dithiocarbazate Schiff base–loaded poly(lactic acid) nanoparticles and analytical validation for drug quantification
Colloid and Polymer Science (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.