Abstract
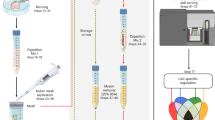
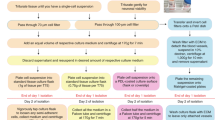
Brain microvascular endothelial cells (BMVECs) have an important role in the constitution of the blood-brain barrier (BBB). The BBB is involved in the disease processes of a number of neurological disorders in which its permeability increases. Isolation of BMVECs could elucidate the mechanism involved in these processes. This protocol describes how to isolate and expand human and mouse BMVECs. The procedure covers brain-tissue dissociation, digestion and cell selection. Cells are selected on the basis of time-responsive differential adhesiveness to a collagen type I–precoated surface. The protocol also describes immunophenotypic characterization, cord formation and functional assays to confirm that these cells in endothelial proliferation medium (EndoPM) have an endothelial origin. The entire technique requires ∼7 h of active time. Endothelial cell clusters are readily visible after 48 h, and expansion of BMVECs occurs over the course of ∼60 d.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Folkman, J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1, 27–31 (1995).
Griffioen, A.W. & Molema, G. Angiogenesis: potentials for pharmacologic intervention in the treatment of cancer, cardiovascular diseases, and chronic inflammation. Pharmacol. Rev. 52, 237–268 (2000).
James, P.B. Pathogenesis of multiple sclerosis: a blood-brain barrier disease. J. R. Soc. Med. 85, 713–714 (1992).
Kalaria, R.N. The blood-brain barrier and cerebral microcirculation in Alzheimer disease. Cerebrovasc. Brain Metab. Rev. 4, 226–260 (1992).
Grammas, P., Martinez, J. & Miller, B. Cerebral microvascular endothelium and the pathogenesis of neurodegenerative diseases. Expert Rev. Mol. Med. 10, 13–19 (2011).
Abbott, N.J., Patabendige, A.A.K., Dolman, D.E.M., Yusof, S.R. & Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 37, 13–25 (2010).
Aird, W.C. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ. Res. 100, 174–190 (2007).
Drewes, L. Molecular architecture of the brain microvasculature. J. Mol. Neurosci. 16, 93–98 (2001).
Bernas, M.J. et al. Establishment of primary cultures of human brain microvascular endothelial cells to provide an in vitro cellular model of the blood-brain barrier. Nat. Protoc. 5, 1265–1272 (2010).
Patabendige, A., Skinner, R.A., Morgan, L. & Joan Abbott, N. A detailed method for preparation of a functional and flexible blood-brain barrier model using porcine brain endothelial cells. Brain Res. 1521 16–30 (2013).
Ribeiro, M.M., Castanho, M.A. & Serrano, I. In vitro blood-brain barrier models–latest advances and therapeutic applications in a chronological perspective. Mini Rev. Med. Chem. 10, 262–270 (2010).
Bowman, P.D. et al. Primary culture of capillary endothelium from rat brain. In Vitro 17, 353–362 (1981).
Bowman, P.D., Ennis, S.R., Rarey, K.E., Betz, A.L. & Goldstein, G.W. Brain microvessel endothelial cells in tissue culture: a model for study of blood-brain barrier permeability. Ann. Neurol. 14, 396–402 (1983).
Dorovini-Zis, K., Prameya, R. & Bowman, P.D. Culture and characterization of microvascular endothelial cells derived from human brain. Lab. Invest. 64, 425–436 (1991).
Manda, K.R., Banerjee, A., Banks, W.A. & Ercal, N. Highly active antiretroviral therapy drug combination induces oxidative stress and mitochondrial dysfunction in immortalized human blood–brain barrier endothelial cells. Free Radical Bio. Med. 50, 801–810 (2011).
Weksler, B.B. et al. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 19, 1872–1874 (2005).
Li, G. et al. Permeability of endothelial and astrocyte cocultures: in vitro blood–brain barrier models for drug delivery studies. Ann. Biomed. Eng. 38, 2499–2511 (2010).
Stannard, A.K., Bradley, N.J. & Owen, J.S. Evaluation of the ECV304 spontaneously transformed HUVEC cell line for adhesion molecule research. Biochem. Soc. Trans. 25, 486S (1997).
Kim, M.H., Jin, E., Zhang, H.Z. & Kim, S.W. Robust angiogenic properties of cultured human peripheral blood-derived CD31+ cells. Int. J. Cardiol. 166, 709–715 (2011).
Sano, Y. & Kanda, T. Isolation and properties of endothelial cells forming the blood-nerve barrier. Methods Mol. Biol. 686, 417–425 (2011).
Van Beijnum, J.R., Rousch, M., Castermans, K., van der Linden, E. & Griffioen, A.W. Isolation of endothelial cells from fresh tissues. Nat. Protoc. 3, 1085–1091 (2008).
Springhorn, J.P. Isolation of human capillary endothelial cells using paramagnetic beads conjugated to anti-PECAM antibodies. Cold Spring Harb. Protoc http://dx.doi.org/10.1101/pdb.prot4479 (2011).
Invernici, G. et al. Human microvascular endothelial cells from different fetal organs demonstrate organ-specific CAM expression. Exp. Cell Res. 308, 273–282 (2005).
Invernici, G. et al. Human fetal aorta contains vascular progenitor cells capable of inducing vasculogenesis, angiogenesis, and myogenesis in vitro and in a murine model of peripheral ischemia. Am J Pathol. 170, 1879–1892 (2007).
Ricci-Vitiani, L. et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature 468, 824–828 (2010).
Navone, S.E. et al. Human and mouse brain–derived endothelial cells require high levels of growth factor medium for their isolation, in vitro maintenance and survival. Vasc. Cell. 5, 10 (2013).
Bachetti, T. & Morbidelli, L. Endothelial cells in culture: a model for studying vascular functions. Pharmacol. Res. 42, 9–19 (2000).
McAuslan, B.R., Hannan, G.N. & Reilly, W. Signals causing change in morphological phenotype, growth mode, and gene expression of vascular endothelial cells. J. Cell. Physiol. 112, 96–106 (1982).
Moncada, S., Palmer, R.M. & Higgs, E.A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 43, 109–142 (1991).
Pardridge, W.M., Boado, R.J. & Farrell, C.R. Brain-type glucose transporter (GLUT-1) is selectively localized to the blood-brain barrier. Studies with quantitative western blotting and in situ hybridization. J. Biol. Chem. 265, 18035–18040 (1990).
Chan, G.N. & Bendayan, R. Molecular and functional characterization of p-glycoprotein in vitro. Methods Mol. Biol. 686, 313–336 (2011).
Male, D.K. Expression and induction of p-glycoprotein-1 on cultured human brain endothelium. J. Cereb. Blood Flow Metab. 29, 1760–1763 (2009).
Yao, G., Lee, T.J., Mori, S., Nevins, J.R. & You, L. A bistable Rb–E2F switch underlies the restriction point. Nat. Cell Biol. 10, 476–482 (2008).
Alessandri, G. et al. Human vasculogenesis ex vivo: embryonal aorta as a tool for isolation of endothelial cell progenitors. Lab. Invest. 81, 875–885 (2001).
Ma, C. & Wang, X.F. In vitro assays for the extracellular matrix protein-regulated extravasation process. Cold Spring Harb. Protoc. http://dx.doi.org/10.1101/pdb.prot5034 (2008).
Acknowledgements
We thank A. Smith for the English review of the paper and A. Canazza, G. Bedini for critical support. This work was supported by the IRCCS Foundation Neurological Institute Carlo Besta (LR8) and by the Italian Ministry of Health (RF2008.22).
Author information
Authors and Affiliations
Contributions
S.E.N. designed and performed experiments, analyzed data and wrote the paper; G.M. gave his contribution to write the paper, supervised and undertook revision of the paper; S.N. performed analysis on cellular cycles and blood-brain barrier; M.S. and A.B. contributed in the isolation of mouse BMVECs; G.A., G.I. and S.C. analyzed molecular data; S.B. and S.S. collected human samples; E.C. performed flow cytometric analysis; E.A.P. supervised and undertook revision of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors have applied for a patent on the EndoPM medium (MI2011000201; PCT and Italian application pending).
Supplementary information
Supplementary Table 1
RT-PCR primer sequences and expected product size. (PDF 67 kb)
Rights and permissions
About this article
Cite this article
Navone, S., Marfia, G., Invernici, G. et al. Isolation and expansion of human and mouse brain microvascular endothelial cells. Nat Protoc 8, 1680–1693 (2013). https://doi.org/10.1038/nprot.2013.107
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2013.107
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.