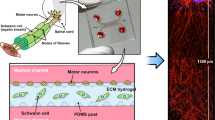
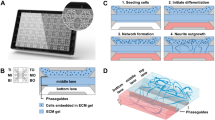
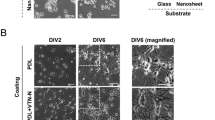
Abstract
Current methods for studying oligodendrocyte myelination using primary neurons are limited by the time, cost and reproducibility of myelination in vitro. Nanofibers with diameters of >0.4 μm fabricated from electrospinning of liquid polystyrene are suitable scaffolds for concentric membrane wrapping by oligodendrocytes. With the advent of aligned electrospinning technology, nanofibers can be rapidly fabricated, standardized, and configured into various densities and patterns as desired. Notably, the minimally permissive culture environment of fibers provides investigators with an opportunity to explore the autonomous oligodendrocyte cellular processes underlying differentiation and myelination. The simplicity of the system is conducive to monitoring oligodendrocyte proliferation, migration, differentiation and membrane wrapping in the absence of neuronal signals. Here we describe protocols for the fabrication and preparation of nanofibers aligned on glass coverslips for the study of membrane wrapping by rodent oligodendrocytes. The entire protocol can be completed within 2 weeks.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Colello, R.J. & Pott, U. Signals that initiate myelination in the developing mammalian nervous system. Mol. Neurobiol. 15, 83–100 (1997).
Ravikumar, M., Jain, S., Miller, R.H., Capadona, J.R. & Selkirk, S.M. An organotypic spinal cord slice culture model to quantify neurodegeneration. J. Neurosci. Methods 211, 280–288 (2012).
Yang, Y., Lewis, R. & Miller, R.H. Interactions between oligodendrocyte precursors control the onset of CNS myelination. Dev. Biol 350, 127–138 (2011).
Lubetzki, C. et al. Even in culture, oligodendrocytes myelinate solely axons. Proc. Natl. Acad. Sci. USA 90, 6820–6824 (1993).
Nash, B. et al. Functional duality of astrocytes in myelination. J. Neurosci. 31, 13028–13038 (2011).
Thomson, C.E. et al. Myelinated, synapsing cultures of murine spinal cord—validation as an in vitro model of the central nervous system. Eur. J. Neurosci 28, 1518–1535 (2008).
Chan, J.R. et al. NGF controls axonal receptivity to myelination by Schwann cells or oligodendrocytes. Neuron 43, 183–191 (2004).
Watkins, T.A., Emery, B., Mulinyawe, S. & Barres, B.A. Distinct stages of myelination regulated by γ-secretase and astrocytes in a rapidly myelinating CNS coculture system. Neuron 60, 555–569 (2008).
Gu, C. & Gu, Y. Clustering and activity tuning of Kv1 channels in myelinated hippocampal axons. J. Biol. Chem. 286, 25835–25847 (2011).
Gardner, A., Jukkola, P. & Gu, C. Myelination of rodent hippocampal neurons in culture. Nat. Protoc. 7, 1774–1782 (2012).
Voyvodic, J.T. Target size regulates calibre and myelination of sympathetic axons. Nature 342, 430–433 (1989).
Lee, S. et al. A culture system to study oligodendrocyte myelination processes using engineered nanofibers. Nat. Methods 9, 917–922 (2012).
Rosenberg, S.S., Kelland, E.E., Tokar, E., De la Torre, A.R. & Chan, J.R. The geometric and spatial constraints of the microenvironment induce oligodendrocyte differentiation. Proc. Natl. Acad. Sci. USA 105, 14662–14667 (2008).
Jagielska, A. et al. Mechanical environment modulates biological properties of oligodendrocyte progenitor cells. Stem Cells Dev. 21, 2905–2914 (2012).
Colognato, H. et al. CNS integrins switch growth factor signalling to promote target-dependent survival. Nat. Cell Biol. 4, 833–841 (2002).
Camara, J. et al. Integrin-mediated axoglial interactions initiate myelination in the central nervous system. J. Cell Biol. 185, 699–712 (2009).
Buttery, P.C. & ffrench-Constant, C. Laminin-2/integrin interactions enhance myelin membrane formation by oligodendrocytes. Mol. Cell Neurosci. 14, 199–212 (1999).
Michailov, G.V. et al. Axonal neuregulin-1 regulates myelin sheath thickness. Science 304, 700–703 (2004).
Hu, X. et al. Bace1 modulates myelination in the central and peripheral nervous system. Nat. Neurosci. 9, 1520–1525 (2006).
Velanac, V. et al. Bace1 processing of NRG1 type III produces a myelin-inducing signal but is not essential for the stimulation of myelination. Glia 60, 203–217 (2012).
Ranscht, B., Clapshaw, P.A., Price, J., Noble, M. & Seifert, W. Development of oligodendrocytes and Schwann cells studied with a monoclonal antibody against galactocerebroside. Proc. Natl. Acad. Sci. USA 79, 2709–2713 (1982).
Sommer, I. & Schachner, M. Monoclonal antibodies (O1 to O4) to oligodendrocyte cell surfaces: an immunocytological study in the central nervous system. Dev. Biol. 83, 311–327 (1981).
Acknowledgements
We thank all the members of the Chan laboratory and the Multiple Sclerosis Research Group at the University of California, San Francisco, for encouragement, advice and insightful discussion. This work was supported by the US National Multiple Sclerosis Society Career Transition Award (TA 3008A2/T), the Harry Weaver Neuroscience Scholar Award (JF 2142-A2/T) and a grant from the US National Institute of Health/National Institute of Neurological Disorders and Stroke (NS062796-02) to J.R.C.
Author information
Authors and Affiliations
Contributions
S.L. performed the experiments and analyzed the data. S.J.T. fabricated the fibers. S.L., S.Y.C.C., J.R.C. and J.M.C. provided intellectual contributions and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Lee, S., Chong, S., Tuck, S. et al. A rapid and reproducible assay for modeling myelination by oligodendrocytes using engineered nanofibers. Nat Protoc 8, 771–782 (2013). https://doi.org/10.1038/nprot.2013.039
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2013.039
This article is cited by
-
Dissecting the complexities of Alzheimer disease with in vitro models of the human brain
Nature Reviews Neurology (2022)
-
Periods of synchronized myelin changes shape brain function and plasticity
Nature Neuroscience (2021)
-
Hypomyelinating leukodystrophies — unravelling myelin biology
Nature Reviews Neurology (2021)
-
An atlas of nano-enabled neural interfaces
Nature Nanotechnology (2019)
-
Approaches to Remyelination Therapies in Multiple Sclerosis
Current Treatment Options in Neurology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.