Abstract
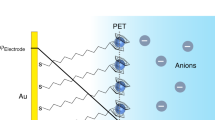
Here we report a protocol to investigate the electron-transfer processes of redox-active biomolecules in biological membranes by electrochemistry using biomimetic hybrid bilayer membranes (HBMs) assembled on gold electrodes. Redox-active head groups, such as the ubiquinone moiety, are embedded in HBMs that contain target molecules, e.g., nicotinamide adenine dinucleotide (NADH). By using this approach, the electron-transfer processes between redox molecules and target biomolecules are mediated by mimicking the redox cycling processes in a natural membrane. Also included is a procedure for in situ surface-enhanced Raman scattering (SERS) to confirm the electrochemically induced conformational changes of the target biomolecules in the HBMs. In addition, each step in constructing the HBMs is characterized by electrochemical impedance spectroscopy (EIS), high-resolution X-ray photoelectron spectroscopy (XPS) and atomic force microscopy (AFM). The time required for the entire protocol is ∼12 h, whereas the electrochemical measurement of electron-transfer processes takes less than 1 h to complete.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Dietz, K.-J. & Pfannschmidt, T. Novel regulators in photosynthetic redox control of plant metabolism and gene expression. Plant Physiol. 155, 1477–1485 (2011).
Foyer, C.H. & Noctor, G. Redox regulation in photosynthetic organisms: signaling, acclimation, and practical implications. Antioxid. Redox Signaling 11, 861–905 (2009).
Zhang, Z. et al. Electron-transfer by domain movement in cytochrome bc 1 . Nature 392, 677–684 (1998).
Joliot, P. & Joliot, A. Cyclic electron transfer in plant leaf. Proc. Natl. Acad. Sci. USA 99, 10209–10214 (2002).
Giorgio, M. et al. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell 122, 221–233 (2005).
Troiano, L. et al. Multiparametric analysis of cells with different mitochondrial membrane potential during apoptosis by polychromatic flow cytometry. Nat. Protoc. 2, 2719–2727 (2007).
Chatterjee, S.N. & Agarwal, S. Liposomes as membrane model for study of lipid peroxidation. Free Radical Biol. Med. 4, 51–72 (1988).
Winterhalter, M. Black lipid membranes. Curr. Opin. Colloid Interface Sci. 5, 250–255 (2000).
Pavinatto, F.J. et al. Probing chitosan and phospholipid interactions using Langmuir and Langmuir-Blodgett films as cell membrane models. Langmuir 23, 7666–7671 (2007).
Czolkos, I., Jesorka, A. & Orwar, O. Molecular phospholipid films on solid supports. Soft Matter 7, 4562–4576 (2011).
Sackman, E. Supported membranes: scientific and practical applications. Science 271, 43–48 (1996).
Castellana, E.T. & Cremer, P.S. Solid supported lipid bilayers: from biophysical studies to sensor design. Surf. Sci. Rep. 61, 429–444 (2006).
Lahiri, J., Kalal, P., Frutos, A.G., Jonas, S.J. & Schaeffler, R. Method for fabricating supported bilayer lipid membranes on gold. Langmuir 16, 7805–7810 (2000).
Cho, N.-J., Frank, C.W., Kasemo, B. & Höök, F. Quartz crystal microbalance with dissipation monitoring of supported lipid bilayers on various substrates. Nat. Protoc. 5, 1096–1106 (2010).
Kraft, M.L., Weber, P.K., Longo, M.L., Hutcheon, I.D. & Boxer, S.G. Phase separation of lipid membranes analyzed with high-resolution secondary ion mass spectrometry. Science 313, 1948–1951 (2006).
Mingeot-Leclercq, M.P., Deleu, M., Brasseur, R. & Dufrêne, Y.F. Atomic force microscopy of supported lipid bilayers. Nat. Protoc. 3, 1654–1659 (2008).
Richter, R.P., Bérat, R. & Brisson, A.R. Formation of solid-supported lipid bilayers: an integrated view. Langmuir 22, 3497–3505 (2006).
Nair, P.M., Salaita, K., Petit, R.S. & Groves, J.T. Using patterned supported lipid membranes to investigate the role of receptor organization in intercellular signaling. Nat. Protoc. 6, 523–539 (2011).
Salamon, Z., Hazzard, J.T. & Tollin, G. Direct measurement of cyclic current-voltage responses of integral membrane proteins at a self-assembled lipid-bilayer-modified electrode: cytochrome f and cytochrome c oxidase. Proc. Natl. Acad. Sci. USA 90, 6420–6423 (1993).
Tanaka, M. & Sackmann, E. Polymer-supported membranes as models of the cell surface. Nature 437, 656–663 (2005).
Burgess, J.D., Rhoten, M.C. & Hawkridge, F.M. Cytochrome c oxidase immobilized in stable supported lipid bilayer membranes. Langmuir 14, 2467–2475 (1998).
Plant, A.L. Supported hybrid bilayer membranes as rugged cell membrane mimics. Langmuir 15, 5128–5135 (1999).
Peng, Z.Q., Tang, J.L., Han, X.J., Wang, E.K. & Dong, S.J. Formation of a supported hybrid bilayer membrane on gold: a sterically enhanced hydrophobic effect. Langmuir 18, 4834–4839 (2002).
Anderson, N.A., Richter, L.J., Stephenson, J.C. & Briggman, K.A. Characterization and control of lipid layer fluidity in hybrid bilayer membranes. J. Am. Chem. Soc. 129, 2094–2100 (2007).
Groves, J.T. & Dustin, M.L. Supported planar bilayers in studies on immune cell adhesion and communication. J. Immunol. Meth. 278, 19–32 (2003).
Hosseini, A. et al. Ferrocene embedded in an electrode-supported hybrid lipid bilayer membrane: a model system for electrocatalysis in a biomimetic environment. Langmuir 26, 17674–17678 (2010).
Groves, J.T. & Boxer, S.G. Micropattern formation in supported lipid membranes. Acc. Chem. Res. 35, 149–157 (2002).
Kett, P.J.N., Casford, M.T.L. & Davies, P.B. Sum frequency generation (SFG) vibrational spectroscopy of planar phosphatidylethanolamine hybrid bilayer membranes under water. Langmuir 26, 9710–9719 (2010).
Twardowski, M. & Nuzzo, R.G. Phase dependent electrochemical properties of polar self-assembled monolayers (SAMs) modified via the fusion of mixed phospholipid vesicles. Langmuir 20, 175–180 (2004).
Dominska, M., Krysinski, P. & Blanchard, G.J. Interrogating interfacial organization in planar bilayer structures. Langmuir 24, 8785–8793 (2008).
Munge, B. et al. Electron-transfer reactions of redox cofactors in spinach photosystem I reaction center protein in lipid films on electrodes. J. Am. Chem. Soc. 125, 12457–12463 (2003).
Salamon, Z., Huang, D., Cramer, W.A. & Tollin, G. Coupled plasmon-waveguide resonance spectroscopy studies of the cytochrome b6f/plastocyanin system in supported lipid bilayer membranes. Biophys. J. 75, 1874–1885 (1998).
Ham, M.-H. et al. Photoelectrochemical complexes for solar energy conversion that chemically and autonomously regenerate. Nat. Chem. 2, 929–936 (2010).
Jeuken, L.J.C. et al. Redox enzymes in tethered membranes. J. Am. Chem. Soc. 128, 1711–1716 (2006).
Ma, W. et al. Reversible redox of NADH and NAD+ at a hybrid lipid bilayer membrane using ubiquinone. J. Am. Chem. Soc. 133, 12366–12369 (2011).
Ma, W. et al. In situ spectroeletrochemistry and cytotoxic activities of natural ubiquinone analogues. Tetrahedron 67, 5990–6000 (2011).
Trumpower, B.L. & Gennis, R.B. Energy transduction by cytochrome complexes in mitochondrial and bacterial respiration: the enzymology of coupling electron-transfer reactions to transmembrane proton translocation. Annu. Rev. Biochem. 63, 675–716 (1994).
Sazanov, L.A. & Hinchliffe, P. Structure of the hydrophilic domain of respiratory complex I from Thermus thermophilus. Science 311, 1430–1436 (2006).
Lee, K.-S., Won, M.-S., Noh, H.-B. & Shim, Y.-B. Triggering the redox reaction of cytochrome c on a biomimetic layer and elimination of interferences for NADH detection. Biomaterials 31, 7827–7835 (2010).
Yoon, J.-H., Lee, K.-S., Yang, J.E., Won, M.-S. & Shim, Y.-B. Electron-transfer kinetics and morphology of cytochrome c at the biomimetic phospholipid layers. J. Electroanal. Chem. 644, 36–43 (2010).
Clapp, P.J., Armitage, B., Roosa, P. & Obrien, D.F. Efficient photoinduced orthogonal energy and electron transfer reactions via phospholipid membrane-bound donors and acceptors. J. Am. Chem. Soc. 116, 9166–9173 (1994).
Li, G. et al. Rigid lipid membranes and nanometer clefts: motifs for the creation of molecular landscapes. Angew. Chem. Int. Ed. 41, 1828–1852 (2002).
Hill, H.D. & Mirkin, C.A. The bio-barcode assay for the detection of protein and nucleic acid targets using DTT-induced ligand exchange. Nat. Protoc. 1, 324–336 (2006).
Steel, A.B., Herne, T.M. & Tarlov, M.J. Electrochemical quantitation of DNA immobilized on gold. Anal. Chem. 70, 4670–4677 (1998).
Hong, H.G. & Park, W. Electrochemical characteristics of hydroquinone-terminated self-assembled monolayers on gold. Langmuir 17, 2485–2492 (2001).
Dieringer, J.A., Lettan, R.B. II, Scheidt, K.A. & Van Duyne, R.P. A frequency domain existence proof of single-molecule surface-enhanced Raman spectroscopy. J. Am. Chem. Soc. 129, 16249–16256 (2007).
Ortiz, R.P. et al. Electronic modulation of dithienothiophene (DTT) as pi-center of D-pi-D chromophores on optical and redox properties: analysis by UV-vis-NIR and Raman spectroscopies combined with electrochemistry and quantum chemical DFT calculations. J. Am. Chem. Soc. 126, 13363–13376 (2004).
Trammell, S.A., Moore, M., Lowy, D. & Lebedev, N. Surface reactivity of the quinone/hydroquinone redox center tethered to gold: comparison of delocalized and saturated bridges. J. Am. Chem. Soc. 130, 5579–5585 (2008).
Green, D.E. & Fleischer, S. The role of lipids in mitochondrial electron transfer and oxidative phosphorylation. Biochim. Biophys. Acta 70, 554–582 (1963).
Rinia, H.A., Bonn, M. & Müller, M. Quantitative multiplex CARS spectroscopy in congested spectral regions. J. Phys. Chem. B 110, 4472–4479 (2006).
Chen, S.P., Hosten, C.M., Vivoni, A., Birke, R.L. & Lombardi, J.R. SERS investigation of NAD+ adsorbed on a silver electrode. Langmuir 18, 9888–9900 (2002).
Siiman, O., Rivellini, R. & Patel, R. Orientation and conformation of NAD+ and NADH adsorbed on colloidal silver. Inorg. Chem. 27, 3940–3949 (1988).
Acknowledgements
This research was supported by the National Science Fund for Distinguished Young Scholars (21125522), the Major Research Plan of the National Natural Science Foundation of China (91027035), the Fundamental Research Funds for the Central Universities (WK1013002) and the 973 Program (2013CB733700).
Author information
Authors and Affiliations
Contributions
W.M. and Y.-T.L. designed and performed all the experiments and analyzed the data. W.M., Y.L.Y., L.X.Q. and H.Z. wrote the manuscript. Z.G. drew and summarized the figures. T.C.S., H.Z., D.-W.L. and H.-Y.C. finalized the preparation of the manuscript. Y.-T.L. supervised all of the work. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figure 1
Cyclic voltammetry of 0.5 mM ubiquinone 0 obtained on a gold electrode in 0.1 M PBS buffer of pH 7.0 at 25 °C. Scan rate is 100 mV s−1. Arrow indicates initial scan direction. The initial scan started from -0.6 V vs. SCE. The voltammetric data is from a multi-cycle experiment. (PDF 276 kb)
Supplementary Figure 2
Cyclic voltammograms of the ubiquinone embedded HBMs system in which NADH or NAD+ was not successfully immobilized in a lipid membrane in 0.1 M PBS buffer of pH 7.0.Q1S-embedded HBMs system (a), Q5S-embedded HBMs system (b), and Q10S-embedded HBMs system (c). Scan rate is 100 mV s−1. Arrow indicates initial scan direction. The initial scan started from -0.50 V vs. SCE for Q1S-embedded HBMs system, -0.60 V vs. SCE for Q5S-embedded HBMs system, and -0.60 V vs. SCE for Q10S-embedded HBMs system. The voltammetric data is from a multi-cycle experiment. (PDF 235 kb)
Supplementary Figure 3
Voltammetric responses of the hexanethiol-embedded HBMs system in the absence (black line) and presence (red line) NADH in lipid membrane in 0.1M PBS buffer of pH 7.0 under nitrogen atmosphere. Arrow indicates initial scan direction. The initial scan started from -0.30 V vs. SCE. The voltammetric data is from a multi-cycle experiment. (Modified with permission from ref. 5). (PDF 229 kb)
Supplementary Figure 4
Voltammetric responses of ubiquinone-embedded HBMs systems (QnS-HBMs) immersed in PBS buffer (pH 7.0) with (red line)/without (black line) NADH under nitrogen atmosphere. (A): Q1S-HBMs (B): Q5S-HBMs; (C): Q10S-HBMs. Arrow indicates initial scan direction. The initial scan started from -0.35 V vs. SCE for Q1S-HBMs system, -0.70 V vs. SCE for Q5S-HBMs system, and -0.70 V vs. SCE for Q10S-HBMs system. The voltammetric data is from a multi-cycle experiment. (Modified with permission from ref. 5). (PDF 91 kb)
Supplementary Figure 5
Nyquist plot for the Faradaic impedance before and after formation of HBMs (with and without NADH) measured on a ubiquinone-functionalized gold electrode. The spectra were recorded in 0.1 M PBS containing 1 mM [Fe(CN)6] 3−/4− as a redox probe, using a frequency range from 100 kHz to 0.1Hz with 10 mV excitation signal. The ESI was measured at formal potential of [Fe(CN)6] 3−/4− vs. SCE. The measured potentials were 0.220 V vs. SCE, 0.214 V vs. SCE, and 0.210 V vs. SCE for Q1S-SAMs, Q1S-HBMs, and Q1S-HBMs-NADH, respectively; The measured potential were 0.225 V vs. SCE, 0.234 V vs. SCE, and 0.236 V vs. SCE for Q5S-SAMs, Q5S-HBMs, and Q5S-HBMs-NADH, respectively; The measured potential were 0.220 V vs. SCE, 0.244 V vs. SCE, and 0.240 V vs. SCE for Q10S-SAMs, Q10S-HBMs, and Q10S-HBMs-NADH, respectively. (Reproduced with permission from ref.5). (PDF 346 kb)
Supplementary Figure 6
AFM images show significant changes in the microscopic features on the electrode surfaces. Shown are (A):Q5S-SAMs; (B):Q5S-HBMs; (C):Q5S-HBMs-NADH. (Reproduced with permission from ref. 5). (PDF 316 kb)
Supplementary Figure 7
High-resolution XPS spectra of S2P for QnS-SAMs, QnS-HBMs, and QnS-HBMs-NADH. Open circles represent experimental raw data, red solid lines are for the total fits, black lines are for the component-fitted peaks, and green lines are for the baselines. (Reproduced with permission from ref. 5). (PDF 306 kb)
Supplementary Figure 8
High-resolution XPS spectra of P2P for QnS-SAMs, QnS-HBMs, and QnS-HBMs-NADH. Open circles represent experimental raw data, red solid lines are for the total fits, black lines are for the component-fitted peaks, and green lines are for the baselines. (Reproduced with permission from ref. 5). (PDF 82 kb)
Supplementary Figure 9
High-resolution XPS spectra of N1s for QnS-SAMs, QnS-HBMs, and QnS-HBMs-NADH. Open circles represent experimental raw data, red solid lines are for the total fits, black lines are for the component-fitted peaks, and green lines are for the baselines. The data of N1s are fit with three components: the quaternary ammonium from lipid of egg phosphatidyl choline (N+, ∼402.5 eV), the non-conjugated nitrogen (∼400.6 eV) and the conjugated nitrogen (∼399.0 eV) from NADH. (Reproduced with permission from ref. 5). (PDF 91 kb)
Rights and permissions
About this article
Cite this article
Ma, W., Ying, YL., Qin, LX. et al. Investigating electron-transfer processes using a biomimetic hybrid bilayer membrane system. Nat Protoc 8, 439–450 (2013). https://doi.org/10.1038/nprot.2013.007
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2013.007
This article is cited by
-
Hybrid bilayer membranes as platforms for biomimicry and catalysis
Nature Reviews Chemistry (2022)
-
Spooling electrochemiluminescence spectroscopy: development, applications and beyond
Nature Protocols (2021)
-
A needle-like reusable surface-enhanced Raman scattering substrate, and its application to the determination of acetamiprid by combining SERS and thin-layer chromatography
Microchimica Acta (2018)
-
Adsorption process of phenothiazine solution in dimethyl sulfoxide on graphite electrodes
Journal of Solid State Electrochemistry (2018)
-
NIR Electrofluorochromic Properties of Aza-Boron-dipyrromethene Dyes
Scientific Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.