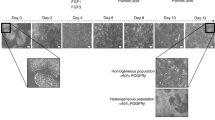
Abstract
Large-scale production of hepatocytes from a variety of genetic backgrounds would be beneficial for drug screening and to provide a source of cells to be used as a substitute for liver transplantation. However, fully functional primary hepatocytes remain difficult to expand in vitro, and circumventing this problem by using an alternative source of cells is desirable. Here we describe a 25-d protocol to direct the differentiation of human pluripotent stem cells into a near-homogenous population of hepatocyte-like cells. As cells progress through this protocol, they express genes in a chronological manner similar to that described during in vivo hepatic development. The protocol relies on culture systems devoid of serum, feeders or complex extracellular matrices, which enable molecular analyses without interference from unknown factors. This approach works efficiently with human embryonic stem cells and human induced pluripotent stem cells and was recently used to model liver diseases in vitro.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Fisher, R.A. & Strom, S.C. Human hepatocyte transplantation: worldwide results. Transplantation 82, 441–449 (2006).
Thomson, J.A. et al. Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 (1998).
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007).
Rashid, S.T. & Vallier, L. Induced pluripotent stem cells—alchemist's tale or clinical reality? Expert Rev. Mol. Med. 12, 25 (2010).
Rashid, S.T. et al. Modeling inherited metabolic disorders of the liver using human induced pluripotent stem cells. J. Clin. Invest. 120, 3127–3136 (2010).
Yusa, K. et al. Targeted gene correction of alpha1-antitrypsin deficiency in induced pluripotent stem cells. Nature 478, 391–394 (2011).
Mikkola, M. et al. Distinct differentiation characteristics of individual human embryonic stem cell lines. BMC Dev. Biol. 6, 40 (2006).
Bader, D. et al. α-Fetoprotein in the early neonatal period—a large study and review of the literature. Clin. Chim. Acta. 349, 15–23 (2004).
Stevens, J.C. et al. Developmental expression of the major human hepatic CYP3A enzymes. J. Pharmacol. Exp. Ther. 307, 573–582 (2003).
Touboul, T. et al. Generation of functional hepatocytes from human embryonic stem cells under chemically defined conditions that recapitulate liver development. Hepatology 51, 1754–1765 (2010).
Brons, I.G. et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature 448, 191–195 (2007).
Yang, Y. et al. The E47 transcription factor negatively regulates CD5 expression during thymocyte development. Proc. Natl. Acad. Sci. USA 101, 3898–3902 (2004).
Vallier, L., Reynolds, D. & Pedersen, R.A. Nodal inhibits differentiation of human embryonic stem cells along the neuroectodermal default pathway. Dev. Biol. 275, 403–421 (2004).
Yamada, T. et al. In vitro differentiation of embryonic stem cells into hepatocyte-like cells identified by cellular uptake of indocyanine green. Stem Cells 20, 146–154 (2002).
Teo, A.K. et al. Pluripotency factors regulate definitive endoderm specification through eomesodermin. Genes Dev. 25, 238–250 (2011).
Rodriguez, R.T. et al. Manipulation of OCT4 levels in human embryonic stem cells results in induction of differential cell types. Exp. Biol. Med. (Maywood) 232, 1368–1380 (2007).
Aguilar-Gallardo, C. et al. Derivation, characterization, differentiation, and registration of seven human embryonic stem cell lines (VAL-3, -4, -5, -6M, -7, -8, and -9) on human feeder. In Vitro Cell Dev. Biol. Anim. 46, 317–326 (2010).
Vallier, L. et al. Signaling pathways controlling pluripotency and early cell fate decisions of human induced pluripotent stem cells. Stem Cells 27, 2655–2666 (2009).
Acknowledgements
This work was funded by a Medical Research Council (MRC) Senior Non-clinical Fellowship (T.T. and L.V.); the Cambridge Hospitals National Institute for Health Research Biomedical Research Center (L.V.); the EU grant LivES and the Evelyn Trust (N.R.F.H.); and the Children's Liver Disease Foundation (C.-P.S.).
Author information
Authors and Affiliations
Contributions
N.R.F.H. and L.V. contributed to the protocol concept, design, interpretation, validation and optimization; C.-P.S. and T.T. contributed to protocol optimization and validation.
Corresponding author
Supplementary information
Supplementary Table 1
Primer sequences for qPCR analyses of genes expressed during differentiation of artificial hepatocytes. (PDF 220 kb)
Supplementary Figure 1
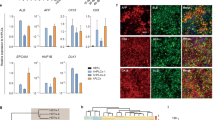
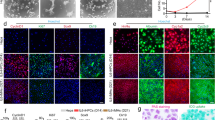
Characterization of hESC-derived hepatocyte-like cells and comparison with primary hepatocytes. (A) Expression of hepatic markers during differentiation of hESCs. qPCR analyses showing the progressive decrease in AFP expression as cells start to mature and at the same time increase expression of the hepatic makers (Albumin and A1AT) during maturation. Primary Hepatocytes (Biopredicts) were used as positive control. Data are presented as the average of 3 biological replicates and error bars indicate standard deviation. (B) ELISA analyses showing increase in Albumin and A1AT secretion during maturation of hepatocytes like cells. Primary Hepatocytes (Biopredicts) were used as positive control. Data are presented as the average of 3 biological replicates and error bars indicate standard deviation. Medium only was used as negative control (C) hPSCs derived hepatocytes cells also display detoxifying activity associated with cytochrome P450 family members such as CyP3A4. This activity can be further induced by chemical such as dexamethasone. (D) Cells also display the ability to take up and metabolise indocyanine green as described by Yamada et el 200214. Scale bar: 200 μm (PDF 433 kb)
Supplementary Figure 2
Comparison of hPSC-derived hepatocytes to human primary fetal hepatocytes. Foetal hepatocytes differentiated from hPSC's share an expression profile very similar to in-vivo derived primary human foetal hepatocytes. AFP, ALB, APOF and CYP3A4 show very similar expression levels when compared between the two cell types, while A1AT does appear to be generally higher along with several other mature metabolic genes such as such as tyrosine amino-transferase (TAT), tryptophan 2,3-dioxygenase (TDO2) and transthyretin (TTR). (PDF 319 kb)
Rights and permissions
About this article
Cite this article
Hannan, N., Segeritz, CP., Touboul, T. et al. Production of hepatocyte-like cells from human pluripotent stem cells. Nat Protoc 8, 430–437 (2013). https://doi.org/10.1038/nprot.2012.153
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2012.153
This article is cited by
-
Cryopreserved cGMP-compliant human pluripotent stem cell-derived hepatic progenitors rescue mice from acute liver failure through rapid paracrine effects on liver cells
Stem Cell Research & Therapy (2024)
-
Extracellular matrix modulates the spatial hepatic features in hepatocyte-like cells derived from human embryonic stem cells
Stem Cell Research & Therapy (2023)
-
The endocannabinoid system promotes hepatocyte progenitor cell proliferation and maturation by modulating cellular energetics
Cell Death Discovery (2023)
-
A dataset of definitive endoderm and hepatocyte differentiations from human induced pluripotent stem cells
Scientific Data (2023)
-
A comprehensive transcriptomic comparison of hepatocyte model systems improves selection of models for experimental use
Communications Biology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.