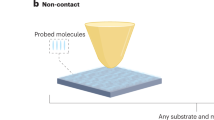
Abstract
Surface-enhanced Raman scattering (SERS) is a powerful fingerprint vibrational spectroscopy with a single-molecule detection limit, but its applications are generally restricted to 'free-electron–like' metal substrates such as Au, Ag and Cu nanostructures. We have invented a shell-isolated nanoparticle-enhanced Raman spectroscopy (SHINERS) technique, using Au-core silica-shell nanoparticles (Au@SiO2 NPs), which makes SERS universally applicable to surfaces with any composition and any morphology. This protocol describes how to prepare shell-isolated nanoparticles (SHINs) with different well-controlled core sizes (55 and 120 nm), shapes (nanospheres, nanorods and nanocubes) and shell thicknesses (1–20 nm). It then describes how to apply SHINs to Pt and Au single-crystal surfaces with different facets in an electrochemical environment, on Si wafer surfaces adsorbed with hydrogen, on ZnO nanorods, and on living bacteria and fruit. With this method, SHINs can be prepared for use in ∼3 h, and each subsequent procedure for SHINERS measurement requires 1–2 h.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
Change history
02 January 2013
In the version of this article initially published online, the Acknowledgments statement was incomplete. It should also have included an acknowledgment of funding from the National Natural Science Foundation of China (NSFC; nos. 21033007, 21021002 and 20825313). The error has been corrected in all versions of the article.
References
Fleischmann, M., Hendra, P.J. & McQuillan, A.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 26, 163–166 (1974).
Albrecht, M.G. & Creighton, J.A. Anomalously intense Raman-spectra of pyridine at a silver electrode. J. Am. Chem. Soc. 99, 5215–5217 (1977).
Jeanmaire, D.L. & Vanduyne, R.P. Surface Raman spectroelectrochemistry .1. heterocyclic, aromatic, and aliphatic-amines adsorbed on anodized silver electrode. J. Electroanal. Chem. 84, 1–20 (1977).
Nie, S.M. & Emery, S.R. Probing single molecules and single nanoparticles by surface-enhanced Raman scattering. Science 275, 1102–1106 (1997).
Kneipp, K. et al. Single molecule detection using surface-enhanced Raman scattering (SERS). Phys. Rev. Lett. 78, 1667–1670 (1997).
Moskovits, M. Surface-enhanced spectroscopy. Rev. Mod. Phys. 57, 783–826 (1985).
Kneipp, K., Moskovits, M. & Kneipp, H. (eds.) Topics in Applied Physics vol. 103; Surface-Enhanced Raman Scattering: Physics and Applications. (Springer, 2006).
Camden, J.P., Dieringer, J.A., Zhao, J. & Van Duyne, R.P. Controlled plasmonic nanostructures for surface-enhanced spectroscopy and sensing. Accounts Chem. Res. 41, 1653–1661 (2008).
Li, J.F. et al. Shell-isolated nanoparticle-enhanced Raman spectroscopy. Nature 464, 392–395 (2010).
Baumberg, J.J. et al. Angle-resolved surface-enhanced Raman scattering on metallic nanostructured plasmonic crystals. Nano Lett. 5, 2262–2267 (2005).
Cao, Y.W.C., Jin, R.C. & Mirkin, C.A. Nanoparticles with Raman spectroscopic fingerprints for DNA and RNA detection. Science 297, 1536–1540 (2002).
Doering, W.E. & Nie, S.M. Spectroscopic tags using dye-embedded nanoparticles and surface-enhanced Raman scattering. Anal. Chem. 75, 6171–6176 (2003).
Chen, Z. et al. Protein microarrays with carbon nanotubes as multicolor Raman labels. Nat. Biotechnol. 26, 1285–1292 (2008).
Jain, P.K., Huang, X.H., El-Sayed, I.H. & El-Sayed, M. Noble metals on the nanoscale: Optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Accounts Chem. Res. 41, 1578–1586 (2008).
Jackson, J.B. & Halas, N.J. Surface-enhanced Raman scattering on tunable plasmonic nanoparticle substrates. Proc. Natl. Acad. Sci. USA 101, 17930–17935 (2004).
Graham, D., Thompson, D.G., Smith, W.E. & Faulds, K. Control of enhanced Raman scattering using a DNA-based assembly process of dye-coded nanoparticles. Nat. Nanotechnol. 3, 548–551 (2008).
Qian, X.M. et al. In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags. Nat. Biotechnol. 26, 83–90 (2008).
Anker, J.N. et al. Biosensing with plasmonic nanosensors. Nat. Mater. 7, 442–453 (2008).
Qin, L.D. et al. Designing, fabricating, and imaging Raman hot spots. Proc. Natl. Acad. Sci. USA 103, 13300–13303 (2006).
Nie, S.M. & Zare, R.N. Optical detection of single molecules. Annu. Rev. Biophys. Biomolec. Struct. 26, 567–596 (1997).
Park, S., Yang, P.X., Corredor, P. & Weaver, M.J. Transition metal-coated nanoparticle films: vibrational characterization with surface-enhanced Raman scattering. J. Am. Chem. Soc. 124, 2428–2429 (2002).
Tian, Z.Q., Ren, B., Li, J.F. & Yang, Z.L. Expanding generality of surface-enhanced Raman spectroscopy with borrowing SERS activity strategy. Chem. Commun. 14, 3514–3534 (2007).
Wu, D.Y., Li, J.F., Ren, B. & Tian, Z.Q. Electrochemical surface-enhanced Raman spectroscopy of nanostructures. Chem. Soc. Rev. 37, 1025–1041 (2008).
Pettinger, B., Ren, B., Picardi, G., Schuster, R. & Ertl, G. Nanoscale probing of adsorbed species by tip-enhanced Raman spectroscopy. Phys. Rev. Lett. 92, 09601 (2004).
Ren, B., Picardi, G., Pettinger, B., Schuster, R. & Ertl, G. Tip-enhanced Raman spectroscopy of benzenethiol adsorbed on Au and Pt single-crystal surfaces. Angew. Chem. In. Ed. 44, 139–142 (2005).
Stockle, R.M., Suh, Y.D., Deckert, V. & Zenobi, R. Nanoscale chemical analysis by tip-enhanced Raman spectroscopy. Chem. Phys. Lett. 318, 131–136 (2000).
Anema, J.R., Li, J.F., Yang, Z.L., Ren, B. & Tian, Z.Q. Shell-isolated nanoparticle-enhanced Raman spectroscopy: expanding the versatility of surface-enhanced Raman scattering. Annu. Rev. Anal. Chem. 4, 129–150 (2011).
Liu, B., Blaszczyk, A., Mayor, M. & Wandlowski, T. Redox-switching in a viologen-type adlayer: An electrochemical shell-isolated nanoparticle enhanced Raman spectroscopy study on Au(111)-(1 x 1) single crystal electrodes. ACS Nano 5, 5662–5672 (2011).
Honesty, N.R. & Gewirth, A.A. Shell-isolated nanoparticle enhanced Raman spectroscopy (SHINERS) investigation of benzotriazole film formation on Cu(100), Cu(111), and Cu(poly). J. Raman Spectrosc. 43, 46–50 (2012).
Li, J.F. et al. Extraordinary enhancement of Raman scattering from pyridine on single crystal Au and Pt electrodes by shell-isolated Au nanoparticles. J. Am. Chem. Soc. 133, 15922–15925 (2011).
Li, J.F. et al. Core-shell nanoparticle based SERS from hydrogen adsorbed on a rhodium(111) electrode. Chem. Commun. 47, 2023–2025 (2011).
Huang, Y.F. et al. Shell-isolated nanoparticle-enhanced Raman spectroscopy of pyridine on smooth silver electrodes. Electrochim. Acta 56, 10652–10657 (2011).
Butcher, D.P., Boulos, S.P., Murphy, C.J., Ambrosio, R.C. & Gewirth, A.A. Face-dependent shell-isolated nanoparticle enhanced Raman spectroscopy of 2,2'-bipyridine on Au(100) and Au(111). J. Phys. Chem. C 116, 5128–5140 (2012).
Graham, D. The next generation of advanced spectroscopy: surface enhanced Raman scattering from metal nanoparticles. Angew. Chem. In. Ed. 49, 9325–9327 (2010).
Guerrero, A.R. & Aroca, R.F. Surface-enhanced fluorescence with shell-isolated nanoparticles (SHINEF). Angew. Chem. In. Ed. 50, 665–668 (2011).
Tian, X.D. et al. SHINERS and plasmonic properties of Au core SiO2 shell nanoparticles with optimal core size and shell thickness. Submitted to. J. Raman Spectrosc. (2012).
Li, S.B. et al. Shell-isolated nanoparticle-enhanced Raman spectroscopy (SHINERS) based on gold-core silica-shell nanorods. Z. Phys. Chem. 225, 775–784 (2011).
Perez-Juste, J., Correa-Duarte, M.A. & Liz-Marzan, L.M. Silica gels with tailored, gold nanorod-driven optical functionalities. Appl. Surf. Sci. 226, 137–143 (2004).
Sendroiu, I.E., Warner, M.E. & Corn, R.M. Fabrication of silica-coated gold nanorods functionalized with DNA for enhanced surface plasmon resonance imaging biosensing applications. Langmuir 25, 11282–11284 (2009).
Zhang, X.Y., Zhao, J., Whitney, A.V., Elam, J.W. & Van Duyne, R.P. Ultrastable substrates for surface-enhanced Raman spectroscopy: Al2O3 overlayers fabricated by atomic layer deposition yield improved anthrax biomarker detection. J. Am. Chem. Soc. 128, 10304–10309 (2006).
Acknowledgements
We thank N.F. Zheng for thoughtful discussions. This work was supported by the Ministry of Science and Technology (MOST) of China (2011YQ030124, 2010IM040100 and 2009CB930703), and by the National Natural Science Foundation of China (NSFC) (21033007, 21021002 and 20825313).
Author information
Authors and Affiliations
Contributions
Z.Q.T., Z.L.W., J.F.L. and B.R. conceived and designed the experiments, analyzed the results and participated in writing the manuscript. J.F.L., X.D.T., S.B.L., J.R.A., Y.D., Y.F.W., Q.Z.C. and Y.M.Z. performed the experiments and analyzed the results. Z.L.Y. contributed the theoretical calculations.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figure 1
Detection of hydrogen adsorption on Pt single-crystal surface. SHINERS spectra of hydrogen adsorbed on Pt(111) at -1.2 V (a), at -1.6 V (b), at -1.9 V (c), without Au@SiO2 NPs at -1.9 V (d), and with the thicker-shell NPs at -1.9 V (e). (PDF 216 kb)
Supplementary Figure 2
Detection of hydrogen adsorption on Si single-crystal surface. SHINERS spectra obtained from Si(111) treated with (a) sulphuric acid, (b) a 30% HF solution, and (c) an oxygen plasma. (PDF 214 kb)
Supplementary Figure 3
Comparison of the adsorption of SCN− on Au single-crystal surface with different facets. SHINERS spectra of SCN− adsorbed on Au(100) (red curves) and Au(111) (black curves) at 0.0 V. (PDF 211 kb)
Supplementary Figure 4
SERS or SHINERS spectra of PATP molecules in different sandwich configurations. (a) Au/PATP/Au NPs, (b) ZnO nanorods/PATP/Au NPs, (c) Au/PATP/Au@SiO2 NPs, and (d) ZnO nanorods/PATP/Au@SiO2 NPs. (PDF 224 kb)
Supplementary Figure 5
SERS or SHINERS study of CO adsorbed on Pt single-crystal surface. The SERS spectrum of CO on Pt(111) at 0.0 V using bare Au NPs (top), and the SHINERS spectrum of CO on Pt(111) at 0.0 V using Au@SiO2 NPs (bottom). (PDF 212 kb)
Supplementary Figure 6
In-situ probing biology structures by SHINERS. (a, b, c) SHINERS spectra obtained from different locations on a sample consisting of yeast cells incubated with Au@SiO2 NPs on a quartz slide. (d) The spectrum of Au@SiO2 NPs, but without yeast cells, on a quartz slide. (e) An ordinary Raman spectrum of yeast cells on a quartz slide. The peaks marked with red asterisks are related to mannoproteins. (PDF 225 kb)
Supplementary Figure 7
In situ detection of a pesticide residue on an orange skin. The Raman signals were collected using a Raman microscope (A) and a portable Raman spectrometer (B). The spectra shown were obtained from a clean orange skin (a), an orange skin contaminated with parathion (b), and an orange skin contaminated with parathion and then modified by addition of Au@SiO2 NPs (c). The Raman spectrum of solid parathion is provided for comparison (d). (PDF 225 kb)
Rights and permissions
About this article
Cite this article
Li, J., Tian, X., Li, S. et al. Surface analysis using shell-isolated nanoparticle-enhanced Raman spectroscopy. Nat Protoc 8, 52–65 (2013). https://doi.org/10.1038/nprot.2012.141
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2012.141
This article is cited by
-
Shell-isolated nanoparticle-enhanced Raman spectroscopy
Nature Reviews Methods Primers (2023)
-
Local cation-tuned reversible single-molecule switch in electric double layer
Nature Communications (2023)
-
Plasmon-enhanced fluorescence for ellagic acid detection based on surface structure of gold nanoparticles
Analytical and Bioanalytical Chemistry (2023)
-
Aptasensor-based assay for dual-readout determination of aflatoxin B1 in corn and wheat via an electrostatic force–mediated FRET strategy
Microchimica Acta (2023)
-
Shell isolated nanoparticle enhanced Raman spectroscopy for mechanistic investigation of electrochemical reactions
Nano Convergence (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.