Abstract
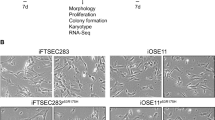
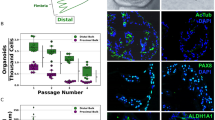
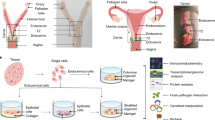
Primary human fallopian tube secretory epithelial cell (FTSEC) cultures are useful for studying normal fallopian tube epithelial biology, as well as for developing models of fallopian tube disease, such as cancer. Because of the limited ability of primary human FTSECs to proliferate in vitro, it is necessary to immortalize them in order to establish a cell line that is suitable for long-term culture and large-scale in vitro experimentation. This protocol describes the isolation of FTSECs from human fallopian tube tissue, conditions for primary FTSEC culture and techniques for establishing immortal FTSEC lines. The entire process, from primary cell isolation to establishment of an immortal cell line, may take up to 2 months. Once established, immortal FTSECs can typically be maintained for at least 30 passages.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Karst, A.M., Levanon, K. & Drapkin, R. Modeling high-grade serous ovarian carcinogenesis from the fallopian tube. Proc. Natl. Acad. Sci. USA 108, 7547–7552 (2011).
Medeiros, F. et al. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am. J. Surg. Pathol. 30, 230–236 (2006).
Lee, Y. et al. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J. Pathol. 211, 26–35 (2007).
Crum, C.P. Intercepting pelvic cancer in the distal fallopian tube: theories and realities. Mol. Oncol. 3, 165–170 (2009).
Przybycin, C.G., Kurman, R.J., Ronnett, B.M., Shih Ie, M. & Vang, R. Are all pelvic (nonuterine) serous carcinomas of tubal origin? Am. J. Surg. Pathol. 34, 1407–1416 (2010).
Crum, C.P. et al. The distal fallopian tube: a new model for pelvic serous carcinogenesis. Curr. Opin. Obstet. Gynecol. 19, 3–9 (2007).
Kurman, R.J. & Shih Ie, M. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer—shifting the paradigm. Hum. Pathol. 42, 918–931 (2011).
Salvador, S. et al. The fallopian tube: primary site of most pelvic high-grade serous carcinomas. Int. J. Gynecol. Cancer 19, 58–64 (2009).
Karst, A.M. & Drapkin, R. Ovarian cancer pathogenesis: a model in evolution. J. Oncol. 2010, 932371 (2010).
Levanon, K., Crum, C. & Drapkin, R. New insights into the pathogenesis of serous ovarian cancer and its clinical impact. J. Clin. Oncol. 26, 5284–5293 (2008).
Greene, M.H., Mai, P.L. & Schwartz, P.E. Does bilateral salpingectomy with ovarian retention warrant consideration as a temporary bridge to risk-reducing bilateral oophorectomy in BRCA1/2 mutation carriers? Am. J. Obstet. Gynecol. 204, 19.e1–19.e6 (2011).
Folkins, A.K., Jarboe, E.A., Roh, M.H. & Crum, C.P. Precursors to pelvic serous carcinoma and their clinical implications. Gynecol. Oncol. 113, 391–396 (2009).
Levanon, K. et al. Primary ex vivo cultures of human fallopian tube epithelium as a model for serous ovarian carcinogenesis. Oncogene 29, 1103–1113 (2010).
Fotheringham, S., Levanon, K. & Drapkin, R. Ex vivo culture of primary human fallopian tube epithelial cells. J. Vis. Exp. doi:10.3791/2728 (2011).
Karst, A.M. & Drapkin, R. The new face of ovarian cancer modeling: better prospects for detection and treatment. F1000 Med. Rep. 3, 22 (2011).
Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 474, 609–615 (2011).
Pearce, C.L. et al. Validating genetic risk associations for ovarian cancer through the International Ovarian Cancer Association Consortium. Br. J. Cancer 100, 412–420 (2009).
Fasching, P.A. et al. Role of genetic polymorphisms and ovarian cancer susceptibility. Mol. Oncol. 3, 171–181 (2009).
Goode, E.L. et al. A genome-wide association study identifies susceptibility loci for ovarian cancer at 2q31 and 8q24. Nat. Genet. 42, 874–879 (2010).
Etemadmoghadam, D. et al. Integrated genome-wide DNA copy number and expression analysis identifies distinct mechanisms of primary chemoresistance in ovarian carcinomas. Clin. Cancer Res. 15, 1417–1427 (2009).
Notaridou, M. et al. Common alleles in candidate susceptibility genes associated with risk and development of epithelial ovarian cancer. Int. J. Cancer 128, 2063–2074 (2011).
Bolton, K.L. et al. Common variants at 19p13 are associated with susceptibility to ovarian cancer. Nat. Genet. 42, 880–884 (2010).
Jazaeri, A.A. et al. Molecular requirements for transformation of fallopian tube epithelial cells into serous carcinoma. Neoplasia 13, 899–911 (2011).
Miller, D.G., Adam, M.A. & Miller, A.D. Gene transfer by retrovirus vectors occurs only in cells that are actively replicating at the time of infection. Mol. Cell Biol. 10, 4239–4242 (1990).
Kanbe, E. & Zhang, D.E. A simple and quick method to concentrate MSCV retrovirus. Blood Cells Mol. Dis. 33, 64–67 (2004).
Laury, A.R. et al. PAX8 reliably distinguishes ovarian serous tumors from malignant mesothelioma. Am. J. Surg. Pathol. 34, 627–635 (2010).
Bowen, N.J. et al. Emerging roles for PAX8 in ovarian cancer and endosalpingeal development. Gynecol. Oncol. 104, 331–337 (2007).
Herbert, B.S., Hochreiter, A.E., Wright, W.E. & Shay, J.W. Nonradioactive detection of telomerase activity using the telomeric repeat amplification protocol. Nat. Protoc. 1, 1583–1590 (2006).
Acknowledgements
Special thanks to the faculty and staff of the Brigham and Women's Hospital Department of Pathology for allocation of tissues. This work was supported by a Canadian Institutes of Health Research Fellowship (A.M.K.), a Kaleidoscope of Hope Young Investigator Research Grant (A.M.K.), US National Institutes of Health grant no. P50 CA105009 (SPORE), the Ovarian Cancer Research Fund (R.D.), the Robert and Debra First Fund (R.D.), the Randi and Joel Cutler Ovarian Cancer Research Fund (R.D.), the Mary Kay Foundation (R.D.), the Sandy Rollman Ovarian Cancer Foundation (R.D.) and the Susan Smith Center for Women's Cancers at the Dana-Farber Cancer Institute (R.D.).
Author information
Authors and Affiliations
Contributions
A.M.K. developed the experimental protocol, performed the experiments and wrote the manuscript. R.D. contributed to the conceptual design of the protocol and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Karst, A., Drapkin, R. Primary culture and immortalization of human fallopian tube secretory epithelial cells. Nat Protoc 7, 1755–1764 (2012). https://doi.org/10.1038/nprot.2012.097
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2012.097
This article is cited by
-
LINE-1 ORF1p as a candidate biomarker in high grade serous ovarian carcinoma
Scientific Reports (2023)
-
L1CAM is required for early dissemination of fallopian tube carcinoma precursors to the ovary
Communications Biology (2022)
-
Preclinical models of epithelial ovarian cancer: practical considerations and challenges for a meaningful application
Cellular and Molecular Life Sciences (2022)
-
Group III phospholipase A2 downregulation attenuated survival and metastasis in ovarian cancer and promotes chemo-sensitization
Journal of Experimental & Clinical Cancer Research (2021)
-
Global miRNA/proteomic analyses identify miRNAs at 14q32 and 3p21, which contribute to features of chronic iron-exposed fallopian tube epithelial cells
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.