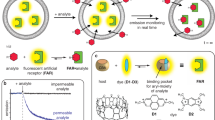
Abstract
The scintillation proximity assay (SPA) is a rapid radioligand binding assay. Upon binding of radioactively labeled ligands (here L-[3H]arginine or D-[3H]glucose) to acceptor proteins immobilized on fluoromicrospheres (containing the scintillant), a light signal is stimulated and measured. The application of SPA to purified, detergent-solubilized membrane transport proteins allows substrate-binding properties to be assessed (e.g., substrate specificity and affinity), usually within 1 d. Notably, the SPA makes it possible to study specific transporters without interference from other cellular components, such as endogenous transporters. Reconstitution of the target transporter into proteoliposomes is not required. The SPA procedure allows high sample throughput and simple sample handling without the need for washing or separation steps: components are mixed in one well and the signal is measured directly after incubation. Therefore, the SPA is an excellent tool for high-throughput screening experiments, e.g., to search for substrates and inhibitors, and it has also recently become an attractive tool for drug discovery.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Udenfriend, S., Gerber, L. & Nelson, N. Scintillation proximity assay: a sensitive and continuous isotopic method for monitoring ligand/receptor and antigen/antibody interactions. Anal. Biochem. 161, 494–500 (1987).
Bosworth, N. & Towers, P. Scintillation proximity assay. Nature 341, 167–168 (1989).
Udenfriend, S., Gerber, L.D., Brink, L. & Spector, S. Scintillation proximity radioimmunoassay utilizing 125I-labeled ligands. Proc. Natl. Acad. Sci. USA 82, 8672–8676 (1985).
Nelson, N. A novel method for the detection of receptors and membrane proteins by scintillation proximity radioassay. Anal. Biochem. 165, 287–293 (1987).
Dallas-Yang, Q., Qureshi, S.A., Xie, D., Zhang, B.B. & Jiang, G. Detection of glucagon-dependent GTPγS binding in high-throughput format. Anal. Biochem. 301, 156–159 (2002).
Ferrer, M. et al. A fully automated [35S]GTPγS scintillation proximity assay for the high-throughput screening of Gi-linked G protein-coupled receptors. Assay Drug Dev. Technol. 1, 261–273 (2003).
DeLapp, N.W. et al. Determination of [35S]guanosine-5′-O-(3-thio)triphosphate binding mediated by cholinergic muscarinic receptors in membranes from Chinese hamster ovary cells and rat striatum using an anti-G protein scintillation proximity assay. J. Pharmacol. Exp. Ther. 289, 946–955 (1999).
Cussac, D., Newman-Tancredi, A., Duqueyroix, D., Pasteau, V. & Millan, M.J. Differential activation of Gq/11 and Gi(3) proteins at 5-hydroxytryptamine(2C) receptors revealed by antibody capture assays: influence of receptor reserve and relationship to agonist-directed trafficking. Mol. Pharmacol. 62, 578–589 (2002).
Newman-Tancredi, A., Cussac, D., Marini, L. & Millan, M.J. Antibody capture assay reveals bell-shaped concentration-response isotherms for h5-HT(1A) receptor-mediated Gα(i3) activation: conformational selection by high-efficacy agonists, and relationship to trafficking of receptor signaling. Mol. Pharmacol. 62, 590–601 (2002).
Odagaki, Y. & Toyoshima, R. Muscarinic acetylcholine receptor-mediated activation of G(q) in rat brain membranes determined by guanosine-5′-O-(3-[(35)S]thio)triphosphate ([(35)S]GTPγS) binding using an anti-G protein scintillation proximity assay. J. Neural. Transm. 119, 525–532 (2011).
DeLapp, N.W. The antibody-capture [(35)S]GTPγS scintillation proximity assay: a powerful emerging technique for analysis of GPCR pharmacology. Trends Pharmacol. Sci. 25, 400–401 (2004).
Quick, M. & Javitch, J.A. Monitoring the function of membrane transport proteins in detergent-solubilized form. Proc. Natl. Acad. Sci. USA 104, 3603–3608 (2007).
Urban, F. Jr . et al. The important role of residue F268 in ligand binding by LXRβ. FEBS Lett. 484, 159–163 (2000).
Jeckelmann, J.-M. et al. Structure and function of the glucose PTS transporter from Escherichia coli. J. Struct. Biol. 176, 395–403 (2011).
Levin, E.J., Quick, M. & Zhou, M. Crystal structure of a bacterial homologue of the kidney urea transporter. Nature 462, 757–761 (2009).
Lu, F. et al. Structure and mechanism of the uracil transporter UraA. Nature 472, 243–246 (2011).
Meury, M. et al. Structure determination of channel and transport proteins by high-resolution microscopy techniques. Biol. Chem. 392, 143–150 (2011).
Piscitelli, C.L., Krishnamurthy, H. & Gouaux, E. Neurotransmitter/sodium symporter orthologue LeuT has a single high-affinity substrate site. Nature 468, 1129–1132 (2010).
Piscitelli, C.L. & Gouaux, E. Insights into transport mechanism from LeuT engineered to transport tryptophan. EMBO J. 31, 228–235 (2011).
Quick, M., Shi, L., Zehnpfennig, B., Weinstein, H. & Javitch, J.A. Experimental conditions can obscure the second high-affinity site in LeuT. Nat. Struct. Mol. Biol. 19, 207–211 (2012).
Zhou, Z. et al. LeuT-desipramine structure reveals how antidepressants block neurotransmitter reuptake. Science 317, 1390–1393 (2007).
Zhou, Z. et al. Antidepressant specificity of serotonin transporter suggested by three LeuT-SSRI structures. Nat. Struct. Mol. Biol. 16, 652–657 (2009).
Glickman, J.F., Schmid, A. & Ferrand, S. Scintillation proximity assays in high-throughput screening. Assay Drug Dev. Technol. 6, 433–455 (2008).
Wu, S. & Liu, B. Application of scintillation proximity assay in drug discovery. BioDrugs 19, 383–392 (2005).
Fang, Y., Kolmakova-Partensky, L. & Miller, C. A bacterial arginine-agmatine exchange transporter involved in extreme acid resistance. J. Biol. Chem. 282, 176–182 (2007).
Harder, D. & Fotiadis, D. Preparation of detergent-solubilized membranes from Escherichia coli. Protoc. Exchange doi:10.1038/protex.2012.033 (2012).
Harder, D. & Fotiadis, D. Purification of His-tagged proteins from detergent-solubilized membranes. Protoc. Exchange doi:10.1038/protex.2012.034 (2012).
Berry, J. & Price-Jones, M. Measurement of radioligand binding by scintillation proximity assay. Methods Mol. Biol. 306, 121–137 (2005).
Carpenter, J.W. et al. Configuring radioligand receptor binding assays for HTS using scintillation proximity assay technology. Methods Mol. Biol. 190, 31–49 (2002).
Shi, L., Quick, M., Zhao, Y., Weinstein, H. & Javitch, J.A. The mechanism of a neurotransmitter:sodium symporter—inward release of Na+ and substrate is triggered by substrate in a second binding site. Mol. Cell 30, 667–677 (2008).
Sorg, G., Schubert, H.D., Buttner, F.H. & Heilker, R. Automated high throughput screening for serine kinase inhibitors using a LEADseeker scintillation proximity assay in the 1536-well format. J. Biomol. Screen 7, 11–19 (2002).
Casagrande, F. et al. Projection structure of a member of the amino acid/polyamine/organocation transporter superfamily. J. Biol. Chem. 283, 33240–33248 (2008).
Porter, A.C. et al. M1 muscarinic receptor signaling in mouse hippocampus and cortex. Brain Res. 944, 82–89 (2002).
Seddon, A.M., Curnow, P. & Booth, P.J. Membrane proteins, lipids and detergents: not just a soap opera. Biochim. Biophys. Acta 1666, 105–117 (2004).
VanAken, T., Foxall-VanAken, S., Castleman, S. & Ferguson-Miller, S. Alkyl glycoside detergents: synthesis and applications to the study of membrane proteins. Methods Enzymol. 125, 27–35 (1986).
Acknowledgements
We are grateful to M. Quick for fruitful discussions, M. Palacin and Z. Ucurum for providing the AdiC constructs, J.-M. Jeckelmann for the IICB clone and J. Gertsch for access to the scintillation counters. This work was supported by the Swiss National Foundation for Scientific Research (grants 31003A_125150 and 31SY30-131038 to D.F.), the European Science Foundation (grant 09-EuroSYNBIO-FP-012 NANOCELL to D.F.), the Novartis Foundation and the National Centre of Competence in Research (NCCR) TransCure.
Author information
Authors and Affiliations
Contributions
D.H. designed and performed experiments, analyzed data and wrote the manuscript; D.F. designed experiments, analyzed data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Harder, D., Fotiadis, D. Measuring substrate binding and affinity of purified membrane transport proteins using the scintillation proximity assay. Nat Protoc 7, 1569–1578 (2012). https://doi.org/10.1038/nprot.2012.090
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2012.090
This article is cited by
-
Functional approaches to the study of G-protein-coupled receptors in postmortem brain tissue: [35S]GTPγS binding assays combined with immunoprecipitation
Pharmacological Reports (2021)
-
IL-13Rα2 uses TMEM219 in chitinase 3-like-1-induced signalling and effector responses
Nature Communications (2016)
-
Scintillation proximity assay (SPA) as a new approach to determine a ligand’s kinetic profile. A case in point for the adenosine A1 receptor
Purinergic Signalling (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.