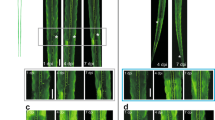
Abstract
This protocol describes an optimized method for direct in vitro monitoring of homo- and heterotypic axon-axon interactions involved in the developmental assembly of neural circuits. The assay exploits a classical example of heterotypic axonal interactions by modeling the sequential extension of spinal motor and somatosensory neuron axons, but the procedure should be readily adaptable to other neuron types. The protocol is based on the rapid isolation and primary culture of genetically identified motor neurons combined with straightforward vital dye labeling and culture of dorsal root ganglion sensory neurons. Subsequently, axonal interactions are directly monitored via live fluorescence microscopy, whereas axon type identities can be unambiguously delineated throughout the experiments. Through chemical compound application or by using neurons derived from genetically engineered mice, the protocol facilitates the dissection of molecular pathways driving the axonal interactions that are crucial for neural pathway and circuit assembly. The whole procedure can be completed in 3 d.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Taylor, A.C. Selectivity of nerve fibers from the dorsal and ventral roots in the development of the frog limb. J. Exp. Zool. 96, 159–185 (1944).
Bate, C.M. Pioneer neurones in an insect embryo. Nature 260, 54–56 (1976).
Kuwada, J.Y. Cell recognition by neuronal growth cones in a simple vertebrate embryo. Science 233, 740–746 (1986).
Sakano, H. Neural map formation in the mouse olfactory system. Neuron 67, 530–542 (2010).
Luo, L. & Flanagan, J.G. Development of continuous and discrete neural maps. Neuron 56, 284–300 (2007).
Komiyama, T. & Luo, L. Development of wiring specificity in the olfactory system. Curr. Opin. Neurobiol. 16, 67–73 (2006).
Hamburger, V. Experimentelle Beiträge zur Entwicklungsphysiologie der Nervenbahnen in der Forschextremität. Rouxs. Arch. Dev. Biol. 119, 47–99 (1929).
Landmesser, L. & Honig, M.G. Altered sensory projections in the chick hind limb following the early removal of motoneurons. Dev. Biol. 118, 511–531 (1986).
Honig, M.G., Lance-Jones, C. & Landmesser, L. The development of sensory projection patterns in embryonic chick hindlimb under experimental conditions. Dev. Biol. 118, 532–548 (1986).
Scott, S.A. Skin sensory innervation patterns in embryonic chick hindlimbs deprived of motoneurons. Dev. Biol. 126, 362–374 (1988).
Swanson, G.J. & Lewis, J. Sensory nerve routes in chick wing buds deprived of motor innervation. J. Embryol. Exp. Morphol. 95, 37–52 (1986).
Tosney, K.W. & Hageman, M.S. Different subsets of axonal guidance cues are essential for sensory neurite outgrowth to cutaneous and muscle targets in the dorsal ramus of the embryonic chick. J. Exp. Zool. 251, 232–244 (1989).
Landmesser, L.T., O'Donovan, M.J. & Honig, M. The response of avian hindlimb motor and sensory neurons to an altered periphery. Prog. Clin. Biol. Res. 110 Part A: 207–216 (1983).
Kapfhammer, J.P. & Raper, J.A. Interactions between growth cones and neurites growing from different neural tissues in culture. J. Neurosci. 7, 1595–1600 (1987).
Kapfhammer, J.P., Grunewald, B.E. & Raper, J.A. The selective inhibition of growth cone extension by specific neurites in culture. J. Neurosci. 6, 2527–2534 (1986).
Bray, D., Wood, P. & Bunge, R.P. Selective fasciculation of nerve fibres in culture. Exp. Cell Res. 130, 241–250 (1980).
Marquardt, T. et al. Coexpressed EphA receptors and ephrin-A ligands mediate opposing actions on growth cone navigation from distinct membrane domains. Cell 121, 127–139 (2005).
Shirasaki, R., Lewcock, J.W., Lettieri, K. & Pfaff, S.L. FGF as a target-derived chemoattractant for developing motor axons genetically programmed by the LIM code. Neuron 50, 841–853 (2006).
Gallarda, B.W. et al. Segregation of axial motor and sensory pathways via heterotypic trans-axonal signaling. Science 320, 233–236 (2008).
Bai, G. et al. Presenilin-dependent receptor processing is required for axon guidance. Cell 144, 106–118 (2011).
Lee, S.K., Jurata, L.W., Funahashi, J., Ruiz, E.C. & Pfaff, S.L. Analysis of embryonic motoneuron gene regulation: derepression of general activators function in concert with enhancer factors. Development 131, 3295–3306 (2004).
deLapeyriere, O. et al. GFR alpha 1 is required for development of distinct subpopulations of motoneuron. J. Neurosci. 20, 4992–5000 (2000).
Nurcombe, V., Hill, M.A., Eagleson, K.L. & Bennett, M.R. Motor neuron survival and neuritic extension from spinal cord explants induced by factors released from denervated muscle. Brain Res. 291, 19–28 (1984).
Heaton, M.B. & Paiva, M. The influence of target tissue age on neurite outgrowth from chick embryo trigeminal motor nucleus explants. Dev. Biol. 116, 314–318 (1986).
Heaton, M.B. & Wayne, D.B. Specific responsiveness of chick trigeminal motor nucleus explants to target-conditioned media. J. Comp. Neurol. 243, 381–387 (1986).
Guthrie, S. & Pini, A. Chemorepulsion of developing motor axons by the floor plate. Neuron 14, 1117–1130 (1995).
Thomson, C.E., Hunter, A.M., Griffiths, I.R., Edgar, J.M. & McCulloch, M.C. Murine spinal cord explants: a model for evaluating axonal growth and myelination in vitro. J. Neurosci. Res. 84, 1703–1715 (2006).
Wang, L., Klein, R., Zheng, B. & Marquardt, T. Anatomical coupling of sensory and motor nerve trajectory via axon tracking. Neuron 71, 263–277 (2011).
Wiese, S. et al. Isolation and enrichment of embryonic mouse motoneurons from the lumbar spinal cord of individual mouse embryos. Nat. Protoc. 5, 31–38 (2010).
Taylor, A.R., Robinson, M.B. & Milligan, C.E. In vitro methods to prepare astrocyte and motoneuron cultures for the investigation of potential in vivo interactions. Nat. Protoc. 2, 1499–1507 (2007).
Calof, A.L. & Reichardt, L.F. Motoneurons purified by cell sorting respond to two distinct activities in myotube-conditioned medium. Dev. Biol. 106, 194–210 (1984).
Buchman, V.L. & Davies, A.M. Different neurotrophins are expressed and act in a developmental sequence to promote the survival of embryonic sensory neurons. Development 118, 989–1001 (1993).
Malin, S.A., Davis, B.M. & Molliver, D.C. Production of dissociated sensory neuron cultures and considerations for their use in studying neuronal function and plasticity. Nat. Protoc. 2, 152–160 (2007).
Pearce, R., Galdzicki, Z. & Rapoport, S.I. Decreased sensitivity to nerve growth factor of dorsal root ganglion neurons cultured from mouse trisomy-16, a model of Down's syndrome. Brain Res. 680, 108–116 (1995).
Arakawa, Y., Sendtner, M. & Thoenen, H. Survival effect of ciliary neurotrophic factor (CNTF) on chick embryonic motoneurons in culture: comparison with other neurotrophic factors and cytokines. J. Neurosci. 10, 3507–3515 (1990).
Bataille, S., Portalier, P., Coulon, P. & Ternaux, J.P. Influence of acetylcholinesterase on embryonic spinal rat motoneurones growth in culture: a quantitative morphometric study. Eur. J. Neurosci. 10, 560–572 (1998).
Camu, W. & Henderson, C.E. Purification of embryonic rat motoneurons by panning on a monoclonal antibody to the low-affinity NGF receptor. J. Neurosci. Methods 44, 59–70 (1992).
Sendtner, M. et al. Isolation and enrichment of embryonic mouse motoneurons from the lumbar spinal cord of individual mouse embryos. Nat. Protoc. 5, 31–38 (2010).
Lemmon, V., Burden, S.M., Payne, H.R., Elmslie, G.J. & Hlavin, M.L. Neurite growth on different substrates: permissive versus instructive influences and the role of adhesive strength. J. Neurosci. 12, 818–826 (1992).
Acknowledgements
We thank A. Klusowski and E. Ling for technical assistance and Olympus-Germany for providing the DSU. This work was supported by the Emmy Noether Program of the Deutsche Forschungsgemeinschaft (DFG), as well as the DFG Research Center for Molecular Physiology of the brain. The ENI-G is a cooperation of the University of Göttingen Medical School and the Max Planck Gesellschaft.
Author information
Authors and Affiliations
Contributions
L.W. designed and executed experiments and analyzed the data. T.M. devised the protocol and supervised the experiments. L.W. and T.M. co-wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Movie 1
Example of a sensory growth cone crossing an interjecting sensory axon. Total duration of movie sequence: 56 min (see Fig. 6a). (AVI 3714 kb)
Supplementary Movie 2
Sensory growth cone (DiI: red) encountering an interjecting motor axon (GFP: green). This encounter leads to reorientation of the sensory axon trajectory, and tracking of the sensory growth cone along the length of the motor axon shaft (see Fig. 6b). Note: tracking is typically accompanied by numerous transient sensory growth cone filopodial-motor axon membrane contacts. Total duration of movie sequence: 189 min. (AVI 12636 kb)
Rights and permissions
About this article
Cite this article
Wang, L., Marquardt, T. Direct live monitoring of heterotypic axon-axon interactions in vitro. Nat Protoc 7, 351–363 (2012). https://doi.org/10.1038/nprot.2011.442
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2011.442
This article is cited by
-
Poly-L-ornithine promotes preferred differentiation of neural stem/progenitor cells via ERK signalling pathway
Scientific Reports (2015)
-
Celsr3 is required in motor neurons to steer their axons in the hindlimb
Nature Neuroscience (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.