Abstract
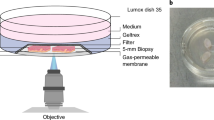
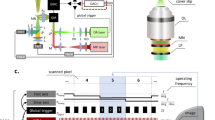
Multiphoton (MP) microscopy enables the direct in vivo visualization, with high spatial and temporal resolution, of fluorescently tagged immune cells, extracellular matrix and vasculature in tissues. This approach, therefore, represents a powerful alternative to traditional methods of assessing immune cell function in the skin, which are mainly based on flow cytometry and histology. Here we provide a step-by-step protocol describing experimental procedures for intravital MP imaging of the mouse ear skin, which can be easily adapted to address many specific skin-related biological questions. We demonstrate the use of this procedure by characterizing the response of neutrophils during cutaneous inflammation, which can be used to perform in-depth analysis of neutrophil behavior in the context of the skin microanatomy, including the epidermis, dermis and blood vessels. Such experiments are typically completed within 1 d, but as the procedures are minimally invasive, it is possible to perform longitudinal studies through repeated imaging.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Cahalan, M.D. & Parker, I. Choreography of cell motility and interaction dynamics imaged by two-photon microscopy in lymphoid organs. Annu. Rev. Immunol. 26, 585–626 (2008).
Coombes, J.L. & Robey, E.A. Dynamic imaging of host-pathogen interactions in vivo. Nat. Rev. Immunol. 10, 353–364 (2010).
Germain, R.N., Miller, M.J., Dustin, M.L. & Nussenzweig, M.C. Dynamic imaging of the immune system: progress, pitfalls and promise. Nat. Rev. Immunol. 6, 497–507 (2006).
Hickman, H.D., Bennink, J.R. & Yewdell, J.W. Caught in the act: intravital multiphoton microscopy of host-pathogen interactions. Cell Host Microbe 5, 13–21 (2009).
Ng, L.G., Mrass, P., Kinjyo, I., Reiner, S.L. & Weninger, W. Two-photon imaging of effector T-cell behavior: lessons from a tumor model. Immunol. Rev. 221, 147–162 (2008).
Roediger, B., Ng, L.G., Smith, A.L., de St Groth, B.F. & Weninger, W. Visualizing dendritic cell migration within the skin. Histochem. Cell Biol. 130, 1131–1146 (2008).
Kissenpfennig, A. et al. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity 22, 643–654 (2005).
Nishibu, A., Ward, B.R., Boes, M. & Takashima, A. Roles for IL-1 and TNFalpha in dynamic behavioral responses of Langerhans cells to topical hapten application. J. Dermatol. Sci. 45, 23–30 (2007).
Nishibu, A. et al. Behavioral responses of epidermal Langerhans cells in situ to local pathological stimuli. J. Invest. Dermatol. 126, 787–796 (2006).
Auffray, C. et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 317, 666–670 (2007).
Weninger, W. et al. Specialized contributions by alpha(1,3)-fucosyltransferase-IV and FucT-VII during leukocyte rolling in dermal microvessels. Immunity 12, 665–676 (2000).
Ng, L.G. et al. Migratory dermal dendritic cells act as rapid sensors of protozoan parasites. PLoS Pathog. 4, e1000222 (2008).
Hoeller, C. et al. In vivo imaging of cutaneous T-cell lymphoma migration to the skin. Cancer Res. 69, 2704–2708 (2009).
Sumaria, N. et al. Cutaneous immunosurveillance by self-renewing dermal {gamma}{delta} T cells. J. Exp. Med. 208, 505–518 (2011).
Ng, L.G. et al. Visualizing the neutrophil response to sterile tissue injury in mouse dermis reveals a three-phase cascade of events. J. Invest. Dermatol. 131, 2058–2068 (2011).
Celli, S., Albert, M.L. & Bousso, P. Visualizing the innate and adaptive immune responses underlying allograft rejection by two-photon microscopy. Nat. Med. 17, 744–749 (2011).
Dudeck, A. et al. Mast cells are key promoters of contact allergy that mediate the adjuvant effects of haptens. Immunity 34, 973–984 (2011).
Matheu, M.P. et al. Imaging of effector memory T cells during a delayed-type hypersensitivity reaction and suppression by Kv1.3 channel block. Immunity 29, 602–614 (2008).
Peters, N.C. et al. In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science 321, 970–974 (2008).
Graham, D.B. et al. ITAM signaling by Vav family Rho guanine nucleotide exchange factors regulates interstitial transit rates of neutrophils in vivo. PLoS ONE 4, e4652 (2009).
Zinselmeyer, B.H., Lynch, J.N., Zhang, X., Aoshi, T. & Miller, M.J. Video-rate two-photon imaging of mouse footpad—a promising model for studying leukocyte recruitment dynamics during inflammation. Inflamm. Res. 57, 93–96 (2008).
Amornphimoltham, P., Masedunskas, A. & Weigert, R. Intravital microscopy as a tool to study drug delivery in preclinical studies. Adv. Drug Deliv. Rev. 63, 119–128 (2011).
Sen, D., Forrest, L., Kepler, T.B., Parker, I. & Cahalan, M.D. Selective and site-specific mobilization of dermal dendritic cells and Langerhans cells by Th1- and Th2-polarizing adjuvants. Proc. Natl. Acad. Sci. USA 107, 8334–8339 (2010).
Hierck, B.P., Iperen, L.V., Gittenberger-De Groot, A.C. & Poelmann, R.E. Modified indirect immunodetection allows study of murine tissue with mouse monoclonal antibodies. J. Histochem. Cytochem. 42, 1499–1502 (1994).
Helft, J. & Merad, M. Isolation of cutaneous dendritic cells. Methods Mol. Biol. 595, 231–233 (2010).
Belkaid, Y., Jouin, H. & Milon, G. A method to recover, enumerate and identify lymphomyeloid cells present in an inflammatory dermal site: a study in laboratory mice. J. Immunol. Methods 199, 5–25 (1996).
Saria, A. & Lundberg, J.M. Evans blue fluorescence: quantitative and morphological evaluation of vascular permeability in animal tissues. J. Neurosci. Methods 8, 41–49 (1983).
Drobizhev, M., Makarov, N.S., Tillo, S.E., Hughes, T.E. & Rebane, A. Two-photon absorption properties of fluorescent proteins. Nat. Methods 8, 393–399 (2011).
Davalos, D. et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 8, 752–758 (2005).
Kim, J.V. & Dustin, M.L. Innate response to focal necrotic injury inside the blood-brain barrier. J. Immunol. 177, 5269–5277 (2006).
Nimmerjahn, A., Kirchhoff, F. & Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318 (2005).
Zoumi, A., Yeh, A. & Tromberg, B.J. Imaging cells and extracellular matrix in vivo by using second-harmonic generation and two-photon excited fluorescence. Proc. Natl. Acad. Sci. USA 99, 11014–11019 (2002).
Beltman, J.B., Maree, A.F. & de Boer, R.J. Analysing immune cell migration. Nat. Rev. Immunol. 9, 789–798 (2009).
Muzumdar, M.D., Tasic, B., Miyamichi, K., Li, L. & Luo, L. A global double-fluorescent Cre reporter mouse. Genesis 45, 593–605 (2007).
Faust, N., Varas, F., Kelly, L.M., Heck, S. & Graf, T. Insertion of enhanced green fluorescent protein into the lysozyme gene creates mice with green fluorescent granulocytes and macrophages. Blood 96, 719–726 (2000).
Townsend, D., Witkop, C.J. Jr. & Mattson, J. Tyrosinase subcellular distribution and kinetic parameters in wild type and C-locus mutant C57BL/6J mice. J. Exp. Zool. 216, 113–119 (1981).
Zhang, J. et al. CD41-YFP mice allow in vivo labeling of megakaryocytic cells and reveal a subset of platelets hyperreactive to thrombin stimulation. Exp. Hematol. 35, 490–499 (2007).
Lindquist, R.L. et al. Visualizing dendritic cell networks in vivo. Nat. Immunol. 5, 1243–1250 (2004).
Jung, S. et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell Biol. 20, 4106–4114 (2000).
Gray, E.E., Suzuki, K. & Cyster, J.G. Cutting edge: identification of a Motile IL-17-Producing {gamma}{delta} T cell population in the dermis. J. Immunol. 186, 6091–6095 (2011).
Unutmaz, D. et al. The primate lentiviral receptor Bonzo/STRL33 is coordinately regulated with CCR5 and its expression pattern is conserved between human and mouse. J. Immunol. 165, 3284–3292 (2000).
Acknowledgements
We thank T. Graf for providing Lysozyme-GFP mice and M. Nussenzweig for providing CD11c-YFP mice. We thank L. Renia and L. Robinson for their careful reading and critical comments on the manuscript. This work was supported by the Agency of Science, Technology and Research (A*STAR), Singapore. This study was also supported by the Australian National Health and Medical Research Council (NHMRC) project grants 570769 and 632706 and a grant from the New South Wales Office of Science and Medical Research (OSMR) to W.W.
Author information
Authors and Affiliations
Contributions
J.L.L., C.C.G., J.L.K. and L.G.N. wrote the first draft and prepared the figures. J.L.L., B.R., J.S.Q., R.J., Y.W. and W.K.C. conducted experiments and further improved the technique. L.G.N. and W.W. developed the methodology for the ear skin imaging.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Close-up image of mouse with an intact left ear (correctly prepared for imaging) and an injured right ear (incorrectly prepared for imaging). Mechanical damage induced during ear preparation can be detected by the leakage of Evans blue. All experiments dealing with live animals were performed in accordance with the relevant animal use and care guidelines and regulations. (TIFF 2894 kb)
Supplementary Fig. 2
Recommended filter and dichroic mirror setups for imaging: Set A: GFP, SHG and Evans blue; Set B: tdTomato, SHG and Evans blue and Set C: YFP, SHG and Evans blue. (EPS 459 kb)
Supplementary Fig. 3
A representative image of a typical setup of an anesthetized mouse on the custom-built intravital mouse ear skin stage. (TIFF 4585 kb)
Supplementary Fig. 4
Multiphoton imaging of immune cells in the skin. Maximal intensity projection of the CD11c-YFP dorsal mouse ear showing (a) Langerhans cells in the epidermis (16 µm stack) and (b) a network of dendritic cells within the highly vascularized dermis (57 µm stack). YFP (yellow, epidermal Langerhans cells and dermal dendritic cells), SHG (blue, collagen fibers) and Evans blue (red, blood vessels), excited at 950 nm with a Ti:Sapphire laser. Imaging of the epidermal layer was performed without hair removal to illustrate the autofluorescence from hairs. The collagen-rich dermis can also be differentiated from the epidermis by the presence of SHG signal. Scan field is 500 × 500 µm and 400 × 400 µm for the epidermis and dermis respectively. Scale bar: 100 µm. All experiments dealing with live animals were performed in accordance with the relevant animal use and care guidelines and regulations. (EPS 14992 kb)
Supplementary Movie 1
Migration of GFP+ cells in the dermis after laser induced injury. A time-lapse sequence of maximum projection (500 x 500 µm scan field, 51 µm stack, 3 µm step size) depicting the migratory patterns of GFP+ cells in the dermis of lysozyme-GFP mice after laser injury. GFP+ cells infiltrate the laser injury site as indicated by intense red signals from the Evans blue leakage. GFP (green, neutrophils), SHG (blue, collagen) and Evans blue (red, blood vessels) were excited with 950 nm, 20 mW at sample, using a tunable Ti:Sapphire laser. The laser injury was induced with 200 mW laser power at 800 nm, 75 × 75 µm scanfield, 242 × 242 pixels, 8.86 µs pixel-1 dwell time. Scale bar: 100 µm. All experiments dealing with live animals were performed in accordance with the relevant animal use and care guidelines and regulations. Elapsed time shown is hh:mm:ss. (MOV 8323 kb)
Supplementary Movie 2
Effects of speckling in pigmented skin. A time-lapse maximum projection of a 3D volume (500 × 500 µm scan field, 48 µm stack, 3 µm step size, 4.31 µs/pixel dwell time). In the pigmented mouse (left), speckling can be first observed within 2.5 minutes of imaging. After 20 minutes, neutrophils start to infiltrate the site where speckling has occurred. This phenomenon was absent in the albino mouse (right). GFP (green, neutrophils), SHG (blue, collagen) and Evans blue (red, blood vessels), were excited with 950 nm, 20 mW at sample, using a tunable Ti:Sapphire laser. Scale bar: 100 µm. All experiments dealing with live animals were performed in accordance with the relevant animal use and care guidelines and regulations. (MOV 6381 kb)
Rights and permissions
About this article
Cite this article
Li, J., Goh, C., Keeble, J. et al. Intravital multiphoton imaging of immune responses in the mouse ear skin. Nat Protoc 7, 221–234 (2012). https://doi.org/10.1038/nprot.2011.438
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2011.438
This article is cited by
-
Real-time observation of neutrophil extracellular trap formation in the inflamed mouse brain via two-photon intravital imaging
Laboratory Animal Research (2022)
-
Determining the immune environment of cutaneous T-cell lymphoma lesions through the assessment of lesional blood drops
Scientific Reports (2021)
-
Labeling and tracking of immune cells in ex vivo human skin
Nature Protocols (2021)
-
Intravital microscopy of dynamic single-cell behavior in mouse mammary tissue
Nature Protocols (2021)
-
Neutrophil extracellular trap-associated RNA and LL37 enable self-amplifying inflammation in psoriasis
Nature Communications (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.