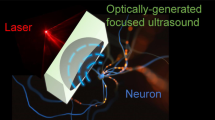
Abstract
Brain stimulation methods are indispensable to the study of brain function. They have also proven effective for treating some neurological disorders. Historically used for medical imaging, ultrasound (US) has recently been shown to be capable of noninvasively stimulating brain activity. Here we provide a general protocol for the stimulation of intact mouse brain circuits using transcranial US, and, using a traditional mouse model of epilepsy, we describe how to use transcranial US to disrupt electrographic seizure activity. The advantages of US for brain stimulation are that it does not necessitate surgery or genetic alteration, but it confers spatial resolutions superior to other noninvasive methods such as transcranial magnetic stimulation. With a basic working knowledge of electrophysiology, and after an initial setup, ultrasonic neuromodulation (UNMOD) can be implemented in less than 1 h. Using the general protocol that we describe, UNMOD can be readily adapted to support a broad range of studies on brain circuit function and dysfunction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Leighton, T.G. What is ultrasound? Prog. Biophys. Mol. Biol. 93, 3–83 (2007).
Harvey, E.N. The effect of high frequency sound waves on heart muscle and other irritable tissues. Am. J. Physiol. 91, 284–290 (1929).
Fry, F.J., Ades, H.W. & Fry, W.J. Production of reversible changes in the central nervous system by ultrasound. Science 127, 83–84 (1958).
Tyler, W.J. et al. Remote excitation of neuronal circuits using low-intensity, low-frequency ultrasound. PLoS ONE 3, e3511 (2008).
Tufail, Y. et al. Transcranial pulsed ultrasound stimulates intact brain circuits. Neuron 66, 681–694 (2010).
Manlapaz, J.S., Astroem, K.E., Ballantine, H.T. Jr. & Lele, P.P. Effects of ultrasonic radiation in experimental focal epilepsy in the cat. Exp. Neurol. 10, 345–356 (1964).
Cazzullo, C.L. & Guareschi, A. Experimental epilepsy produced by ultrasonics. I. Semeiological, electroencephalographic and anatomicopathological observations following application of low dosage of ultrasonics on guinea pig encephalon with intact theca. Riv. Patol. Nerv. Ment. 74, 545–572 (1953).
Allegranza, A. Effect of anticonvulsant and neuroplegic drugs on experimental epilepsy induced with ultrasonics. Rev. Neurol. (Paris) 94, 395–399 (1956).
Min, B.K. et al. Focused ultrasound-mediated suppression of chemically-induced acute epileptic EEG activity. BMC Neurosci. 12, 23 (2011).
O'Brien, W.D. Jr. Ultrasound-biophysics mechanisms. Prog. Biophys. Mol. Biol. 93, 212–255 (2007).
Dinno, M.A. et al. The significance of membrane changes in the safe and effective use of therapeutic and diagnostic ultrasound. Phys. Med. Biol. 34, 1543–1552 (1989).
ter Haar, G. Therapeutic applications of ultrasound. Prog. Biophys. Mol. Biol. 93, 111–129 (2007).
Dalecki, D. Mechanical bioeffects of ultrasound. Annu. Rev. Biomed. Eng. 6, 229–248 (2004).
Fritsch, G. & Hitzig, E. Über die elektrische Erregbarkeit des Grosshirns. Arch. Anat. Physiol. 37, 300–332 (1870).
Miesenbock, G. The optogenetic catechism. Science 326, 395–399 (2009).
Deisseroth, K. Optogenetics. Nat. Methods 8, 26–29 (2011).
Kringelbach, M.L., Jenkinson, N., Owen, S.L. & Aziz, T.Z. Translational principles of deep brain stimulation. Nat. Rev. Neurosci. 8, 623–635 (2007).
Schwalb, J.M. & Hamani, C. The history and future of deep brain stimulation. Neurotherapeutics 5, 3–13 (2008).
Histed, M.H., Bonin, V. & Reid, R.C. Direct activation of sparse, distributed populations of cortical neurons by electrical microstimulation. Neuron 63, 508–522 (2009).
Ranck, J.B. Jr. Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res. 98, 417–440 (1975).
Stoney, S.D. Jr., Thompson, W.D. & Asanuma, H. Excitation of pyramidal tract cells by intracortical microstimulation: effective extent of stimulating current. J. Neurophysiol. 31, 659–669 (1968).
Tolias, A.S. et al. Mapping cortical activity elicited with electrical microstimulation using FMRI in the macaque. Neuron 48, 901–911 (2005).
Grill, W.M., Norman, S.E. & Bellamkonda, R.V. Implanted neural interfaces: biochallenges and engineered solutions. Annu. Rev. Biomed. Eng. 11, 1–24 (2009).
Payne, N.A. & Prudic, J. Electroconvulsive therapy: Part I. A perspective on the evolution and current practice of ECT. J. Psychiatr. Practice 15, 346–368 (2009).
Wagner, T., Valero-Cabre, A. & Pascual-Leone, A. Noninvasive human brain stimulation. Annu. Rev. Biomed. Eng. 9, 527–565 (2007).
Barker, A.T. The history and basic principles of magnetic nerve stimulation. Electroencephalogr. Clin. Neurophysiol. Suppl. 51, 3–21 (1999).
Fregni, F. & Pascual-Leone, A. Technology insight: noninvasive brain stimulation in neurology-perspectives on the therapeutic potential of rTMS and tDCS. Nat. Clin. Practice Neurol. 3, 383–393 (2007).
Hallett, M. Transcranial magnetic stimulation: a primer. Neuron 55, 187–199 (2007).
Zhang, Y.P. et al. Use of magnetic stimulation to elicit motor evoked potentials, somatosensory evoked potentials, and H-reflexes in non-sedated rodents. J. Neurosci. Methods 165, 9–17 (2007).
Ji, R.R. et al. Repetitive transcranial magnetic stimulation activates specific regions in rat brain. Proc. Natl. Acad. Sci. USA 95, 15635–15640 (1998).
Cambiaghi, M. et al. Brain transcranial direct current stimulation modulates motor excitability in mice. Eur. J. Neurosci. 31, 704–709 (2010).
Zhang, S., Yin, L. & Fang, N. Focusing ultrasound with an acoustic metamaterial network. Phys. Rev. Lett, 102, 194301–194304 (2009).
Li, J., Fok, L., Yin, X., Bartal, G. & Zhang, X. Experimental demonstration of an acoustic magnifying hyperlens. Nat. Mater. 8, 931–934 (2009).
Ayling, O.G., Harrison, T.C., Boyd, J.D., Goroshkov, A. & Murphy, T.H. Automated light-based mapping of motor cortex by photoactivation of channelrhodopsin-2 transgenic mice. Nat. Methods 6, 219–224 (2009).
Luft, A.R. et al. Transcranial magnetic stimulation in the rat. Exp. Brain Res. 140, 112–121 (2001).
Nielsen, J.B., Perez, M.A., Oudega, M., Enriquez-Denton, M. & Aimonetti, J.M. Evaluation of transcranial magnetic stimulation for investigating transmission in descending motor tracts in the rat. Eur. J.Neurosci. 25, 805–814 (2007).
Heimburg, T. Lipid ion channels. Biophys. Chem. 150, 2–22 (2010).
Heimburg, T. & Jackson, A.D. On soliton propagation in biomembranes and nerves. Proc. Natl. Acad. Sci. USA 102, 9790–9795 (2005).
Griesbauer, J., Wixforth, A. & Schneider, M.F. Wave propagation in lipid monolayers. Biophys. J. 97, 2710–2716 (2009).
Yoo, S.S. et al. Focused ultrasound modulates region-specific brain activity. Neuroimage 56, 1267–1275 (2011).
Ang, E.S. Jr., Gluncic, V., Duque, A., Schafer, M.E. & Rakic, P. Prenatal exposure to ultrasound waves impacts neuronal migration in mice. Proc. Natl. Acad. Sci. USA 103, 12903–12910 (2006).
Martin, E., Jeanmonod, D., Morel, A., Zadicario, E. & Werner, B. High intensity focused ultrasound for non-invaisve functional neurosurgery. Ann. Neurol. 66, 858–861 (2009).
Hynynen, K. & Clement, G. Clinical applications of focused ultrasound-the brain. Int. J. Hyperthermia 23, 193–202 (2007).
Altland, O.D., Dalecki, D., Suchkova, V.N. & Francis, C.W. Low-intensity ultrasound increases endothelial cell nitric oxide synthase activity and nitric oxide synthesis. J. Thromb. Haemost. 2, 637–643 (2004).
Claes, L. & Willie, B. The enhancement of bone regeneration by ultrasound. Prog. Biophys. Mol. Biol. 93, 384–398 (2007).
Ebisawa, K. et al. Ultrasound enhances transforming growth factor beta-mediated chondrocyte differentiation of human mesenchymal stem cells. Tissue Eng. 10, 921–929 (2004).
Hsu, H.C. et al. Ultrasound induces cyclooxygenase-2 expression through integrin, integrin-linked kinase, Akt, NF-kappaB and p300 pathway in human chondrocytes. Cell Signal. 19, 2317–2328 (2007).
Reher, P., Doan, N., Bradnock, B., Meghji, S. & Harris, M. Effect of ultrasound on the production of IL-8, basic FGF and VEGF. Cytokine 11, 416–423 (1999).
Sant'Anna, E.F., Leven, R.M., Virdi, A.S. & Sumner, D.R. Effect of low intensity pulsed ultrasound and BMP-2 on rat bone marrow stromal cell gene expression. J. Orthop. Res. 23, 646–652 (2005).
Sena, K., Leven, R.M., Mazhar, K., Sumner, D.R. & Virdi, A.S. Early gene response to low-intensity pulsed ultrasound in rat osteoblastic cells. Ultrasound Med. Biol. 31, 703–708 (2005).
Mihran, R.T., Barnes, F.S. & Wachtel, H. Transient modification of nerve excitability in vitro by single ultrasound pulses. Biomed. Sci. Instrum. 26, 235–246 (1990).
White, P.J., Clement, G.T. & Hynynen, K. Longitudinal and shear mode ultrasound propagation in human skull bone. Ultrasound Med. Biol. 32, 1085–1096 (2006).
White, P.J., Clement, G.T. & Hynynen, K. Local frequency dependence in transcranial ultrasound transmission. Phys. Med. Biol. 51, 2293–2305 (2006).
Hayner, M. & Hynynen, K. Numerical analysis of ultrasonic transmission and absorption of oblique plane waves through the human skull. J. Acoust. Soc. Am. 110, 3319–3330 (2001).
Kinoshita, M., McDannold, N., Jolesz, F.A. & Hynynen, K. Targeted delivery of antibodies through the blood-brain barrier by MRI-guided focused ultrasound. Biochem. Biophys. Res. Commun. 340, 1085–1090 (2006).
Larrat, B. et al. MR-guided transcranial brain HIFU in small animal models. Phys. Med. Biol. 55, 365–388 (2010).
Hynynen, K., McDannold, N., Sheikov, N.A., Jolesz, F.A. & Vykhodtseva, N. Local and reversible blood-brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. Neuroimage 24, 12–20 (2005).
Hynynen, K. et al. Pre-clinical testing of a phased array ultrasound system for MRI-guided noninvasive surgery of the brain—a primate study. Eur. J. Radiol. 59, 149–156 (2006).
Hynynen, K. et al. 500-element ultrasound phased array system for noninvasive focal surgery of the brain: a preliminary rabbit study with ex vivo human skulls. Magn. Reson. Med. 52, 100–107 (2004).
Hynynen, K. & Jolesz, F.A. Demonstration of potential noninvasive ultrasound brain therapy through an intact skull. Ultrasound Med. Biol. 24, 275–283 (1998).
Clement, G.T. & Hynynen, K. A non-invasive method for focusing ultrasound through the human skull. Phys. Med. Biol. 47, 1219–1236 (2002).
Bartholow, R. Medical Electricity: a Practical Treatise on the Applications of Electricity to Medicine and Surgery 2nd edn, (Henry C. Lea's Son & Co., 1882).
Barker, A.T., Jalinous, R. & Freeston, I.L. Non-invasive magnetic stimulation of human motor cortex. Lancet 1, 1106–1107 (1985).
Aravanis, A.M. et al. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J. Neural Eng. 4, S143–S156 (2007).
Penfield, W. & Jasper, H.H. Epilepsy and the Functional Anatomy of the Human Brain (J & A Churchill, 1954).
Hamani, C., Andrade, D., Hodaie, M., Wennberg, R. & Lozano, A. Deep brain stimulation for the treatment of epilepsy. Int. J. Neural Syst. 19, 213–226 (2009).
Jobst, B.C. Electrical stimulation in epilepsy: vagus nerve and brain stimulation. Curr. Treat. Opt. Neurol. 12, 443–453 (2010).
Theodore, W.H. & Fisher, R.S. Brain stimulation for epilepsy. Lancet Neurol. 3, 111–118 (2004).
Morrell, M. Brain stimulation for epilepsy: can scheduled or responsive neurostimulation stop seizures? Curr. Opin. Neurol. 19, 164–168 (2006).
Saillet, S. et al. Manipulating the epileptic brain using stimulation: a review of experimental and clinical studies. Epileptic Disord. 11, 100–112 (2009).
Boon, P. et al. Deep brain stimulation in patients with refractory temporal lobe epilepsy. Epilepsia 48, 1551–1560 (2007).
Liebetanz, D. et al. Anticonvulsant effects of transcranial direct-current stimulation (tDCS) in the rat cortical ramp model of focal epilepsy. Epilepsia 47, 1216–1224 (2006).
Jennum, P. & Klitgaard, H. Repetitive transcranial magnetic stimulations of the rat. Effect of acute and chronic stimulations on pentylenetetrazole-induced clonic seizures. Epilepsy Res. 23, 115–122 (1996).
Sharma, A.K. et al. Mesial temporal lobe epilepsy: pathogenesis, induced rodent models and lesions. Toxicol. Pathol. 35, 984–999 (2007).
NEMA. Acoustic Output Measurement Standard For Diagnostic Ultrasound Equipment (National Electrical Manufacturers Association, 2004).
Franklin, K.B.J. & Paxinos, G. The Mouse Brain in Stereotaxic Coordinates 3rd edn, (Academic Press, 2007).
Racine, R.J. Modification of seizure activity by electrical stimulation. I. After-discharge threshold. Electroencephalogr. Clin. Neurophysiol. 32, 269–279 (1972).
Shealy, C.N. & Henneman, E. Reversible effects of ultrasound on spinal reflexes. Arch. Neurol. 374–386 (1962).
Gavrilov, L.R. et al. The effect of focused ultrasound on the skin and deep nerve structures of man and animal. Prog. Brain Res. 43, 279–292 (1976).
Tsirulnikov, E.M. et al. Use of amplitude-modulated focused ultrasound for diagnosis of hearing disorders. Ultrasound Med. Biol. 14, 277–285 (1988).
Foster, K.R. & Wiederhold, M.L. Auditory responses in cats produced by pulsed ultrasound. J. Acoust. Soc. Am. 63, 1199–1205 (1978).
Magee, T.R. & Davies, A.H. Auditory phenomena during transcranial Doppler insonation of the basilar artery. J. Ultrasound Med. 12, 747–750 (1993).
Tsui, P.H., Wang, S.H. & Huang, C.C. In vitro effects of ultrasound with different energies on the conduction properties of neural tissue. Ultrasonics 43, 560–565 (2005).
Mihran, R.T., Barnes, F.S. & Wachtel, H. Temporally-specific modification of myelinated axon excitability in vitro following a single ultrasound pulse. Ultrasound Med. Biol. 16, 297–309 (1990).
Foley, J.L., Little, J.W. & Vaezy, S. Image-guided high-intensity focused ultrasound for conduction block of peripheral nerves. Ann. Biomed. Eng. 35, 109–119 (2007).
Bachtold, M.R., Rinaldi, P.C., Jones, J.P., Reines, F. & Price, L.R. Focused ultrasound modifications of neural circuit activity in a mammalian brain. Ultrasound Med. Biol. 24, 557–565 (1998).
Rinaldi, P.C., Jones, J.P., Reines, F. & Price, L.R. Modification by focused ultrasound pulses of electrically evoked responses from an in vitro hippocampal preparation. Brain Res. 558, 36–42 (1991).
Ludwig, G.D. The velocity of sound through tissues and the acoustic impedance of tissues. J. Acoust. Soc. Am. 22, 862–866 (1950).
Turker, K.S. Electromyography: some methodological problems and issues. Phys. Ther. 73, 698–710 (1993).
Whelan, P.J. Electromyogram recordings from freely moving animals. Methods 30, 127–141 (2003).
Acknowledgements
Support for this work was provided by start-up funds from Arizona State University to W.J.T. and Department of Defense grants from the US Army Research, Development, and Engineering Command (RDECOM W911NF-09-0431) and a Defense Advanced Research Projects Agency Young Faculty Award (DARPA N66001-10-1-4032) to W.J.T.
Author information
Authors and Affiliations
Contributions
Y.T., A.Y., S.P. and W.J.T. designed and conducted the experimental procedures. Y.T., A.Y., M.M.L. and W.J.T. analyzed and interpreted data from the experiments. Y.T., A.Y., S.P., M.M.L. and W.J.T. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
W.J.T. is a cofounder of SynSonix.
Supplementary information
Supplementary Video 1
Overview of ultrasonic neuromodulation rig and equipment (MOV 30900 kb)
Supplementary Video 2
Disruption of seizure activity with TPU (MOV 8962 kb)
Supplementary Video 3
Disruption of electrographic seizure activity with continuous wave UNMOD waveforms (MOV 1490 kb)
Rights and permissions
About this article
Cite this article
Tufail, Y., Yoshihiro, A., Pati, S. et al. Ultrasonic neuromodulation by brain stimulation with transcranial ultrasound. Nat Protoc 6, 1453–1470 (2011). https://doi.org/10.1038/nprot.2011.371
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2011.371
This article is cited by
-
Ultrasound neuromodulation of cultured hippocampal neurons
Biomedical Engineering Letters (2024)
-
Exploring the dynamical transitions on an epileptic hippocampal network model and its modulation strategy based on transcranial magneto-acoustical stimulation
Nonlinear Dynamics (2024)
-
Low-intensity transcranial ultrasound stimulation modulates neural activities in mice under propofol anaesthesia
BMC Neuroscience (2023)
-
Functional nanoparticle-enabled non-genetic neuromodulation
Journal of Nanobiotechnology (2023)
-
Transcranial focused ultrasound-mediated unbinding of phenytoin from plasma proteins for suppression of chronic temporal lobe epilepsy in a rodent model
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.