Abstract
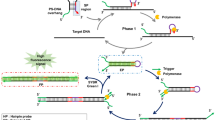
This protocol describes a new and rapid isothermal reaction process designed to amplify and detect a specific DNA sequence in purified DNA extracted from cultured cells. The protocol uses a DNA nanomachine that comprises two molecular switches that function in concert to isothermally amplify and detect a DNA target. First, a molecular beacon detection switch is 'activated' only if a DNA target sequence is present. A DNA primer and DNA polymerase are used to lock the beacon in an activated conformation. Second, an amplification and signal-transduction switch is initiated following successful activation. A nicking endonuclease and the DNA polymerase are used to replicate the DNA target. Both switches operate simultaneously at 40 °C in a single reaction to rapidly generate multiple copies of the DNA target in a cyclic polymerization reaction. This protocol enables femtomole amounts of a DNA target to be reproducibly amplified and detected in <40 min. We demonstrate the successful use of this protocol in assays containing synthetic DNA components and purified DNA extracted from biological samples.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Mullis, K. et al. Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction. Cold Spring Harb. Symp. Quant. Biol. 51 (Part 1): 263–273 (1986).
Gill, P. & Ghaemi, A. Nucleic acid isothermal amplification technologies: a review. Nucleosides Nucleotides Nucleic Acids 27, 224–243 (2008).
Compton, J. Nucleic acid sequence-based amplification. Nature 350, 91–92 (1991).
Kwoh, D.Y. et al. Transcription-based amplification system and detection of amplified human immunodeficiency virus type 1 with a bead-based sandwich hybridization format. Proc. Natl. Acad. Sci. USA 86, 1173–1177 (1989).
Wharam, S.D. et al. Specific detection of DNA and RNA targets using a novel isothermal nucleic acid amplification assay based on the formation of a three-way junction structure. Nucleic Acids Res. 29, E54–E54 (2001).
Guatelli, J.C. et al. Isothermal, in vitro amplification of nucleic acids by a multienzyme reaction modeled after retroviral replication. Proc. Natl. Acad. Sci. USA 87, 1874–1878 (1990).
Notomi, T. et al. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 28, E63 (2000).
Kiesling, T. et al. Sequence specific detection of DNA using nicking endonuclease signal amplification (NESA). Nucleic Acids Res. 35, e117 (2007).
Vincent, M., Xu, Y. & Kong, H. Helicase-dependent isothermal DNA amplification. EMBO Rep. 5, 795–800 (2004).
Walker, G.T., Little, M.C., Nadeau, J.G. & Shank, D.D. Isothermal in vitro amplification of DNA by a restriction enzyme/DNA polymerase system. Proc. Natl. Acad. Sci. USA 89, 392–396 (1992).
Fire, A. & Xu, S.Q. Rolling replication of short DNA circles. Proc. Natl. Acad. Sci. USA 92, 4641–4645 (1995).
Kurn, N. et al. Novel isothermal, linear nucleic acid amplification systems for highly multiplexed applications. Clin. Chem. 51, 1973–1981 (2005).
Li, J.J., Chu, Y., Lee, B.Y. & Xie, X.S. Enzymatic signal amplification of molecular beacons for sensitive DNA detection. Nucleic Acids Res. 36, e36 (2008).
Sando, S., Narita, A., Abe, K. & Aoyama, Y. Doubly catalytic sensing of HIV-1-related CCR5 sequence in prokaryotic cell-free translation system using riboregulator-controlled luciferase activity. J. Am. Chem. Soc. 127, 5300–5301 (2005).
Van Ness, J., Van Ness, L.K. & Galas, D.J. Isothermal reactions for the amplification of oligonucleotides. Proc. Natl. Acad. Sci. USA 100, 4504–4509 (2003).
Weizmann, Y., Cheglakov, Z., Pavlov, V. & Willner, I. Autonomous fueled mechanical replication of nucleic acid templates for the amplified optical detection of DNA. Angew Chem. Int. Ed. Engl. 45, 2238–2242 (2006).
Yi, J., Zhang, W. & Zhang, D.Y. Molecular Zipper: a fluorescent probe for real-time isothermal DNA amplification. Nucleic Acids Res. 34, e81 (2006).
Connolly, A.R. & Trau, M. Isothermal detection of DNA by beacon-assisted detection amplification. Angew Chem. Int. Ed. Engl. 49, 2720–2723 (2010).
Acknowledgements
We gratefully acknowledge funding and support from the NBCF through the National Collaborative Breast Cancer Research Grant.
Author information
Authors and Affiliations
Contributions
A.R.C. conceived the idea. A.R.C. and M.T. designed and reviewed the entire experimental program. A.R.C. carried out the experiments and wrote the paper. All of the work was carried out within the laboratory of M.T.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Connolly, A., Trau, M. Rapid DNA detection by beacon-assisted detection amplification. Nat Protoc 6, 772–778 (2011). https://doi.org/10.1038/nprot.2011.326
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2011.326
This article is cited by
-
Nucleic Acids Analysis
Science China Chemistry (2021)
-
Quadratic isothermal amplification for the detection of microRNA
Nature Protocols (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.