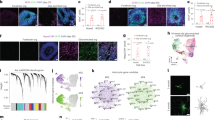
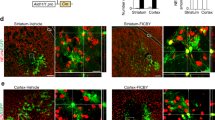
Abstract
Instructing glial cells to generate neurons may prove to be a strategy to replace neurons that have degenerated. Here, we describe a robust protocol for the efficient in vitro conversion of postnatal astroglia from the mouse cerebral cortex into functional, synapse-forming neurons. This protocol involves two steps: (i) expansion of astroglial cells (7 d) and (ii) astroglia-to-neuron conversion induced by persistent and strong retroviral expression of Neurog2 (encoding neurogenin-2) or Mash1 (also referred to as achaete-scute complex homolog 1 or Ascl1) and/or distal-less homeobox 2 (Dlx2) for generation of glutamatergic or GABAergic neurons, respectively (7–21 d for different degrees of maturity). Our protocol of astroglia-to-neuron conversion by a single neurogenic transcription factor provides a stringent experimental system to study the specification of a selective neuronal subtype, thus offering an alternative to the use of embryonic or neural stem cells. Moreover, it can be a useful model for studies of lineage conversion from non-neuronal cells, with potential for brain regenerative medicine.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kriegstein, A. & Alvarez-Buylla, A. The glial nature of embryonic and adult neural stem cells. Annu. Rev. Neurosci. 32, 149–184 (2009).
Hirabayashi, Y. et al. Polycomb limits the neurogenic competence of neural precursor cells to promote astrogenic fate transition. Neuron 63, 600–613 (2009).
Heins, N. et al. Glial cells generate neurons: the role of the transcription factor Pax6. Nat. Neurosci. 5, 308–315 (2002).
Heinrich, C. et al. Directing astroglia from the cerebral cortex into subtype specific functional neurons. PLoS Biol. 8, e1000373 (2010).
Berninger, B. et al. Functional properties of neurons derived from in vitro reprogrammed postnatal astroglia. J. Neurosci. 27, 8654–8664 (2007).
Campbell, K. & Götz, M. Radial glia: multi-purpose cells for vertebrate brain development. Trends Neurosci. 25, 235–238 (2002).
Pinto, L. & Götz, M. Radial glial cell heterogeneity—the source of diverse progeny in the CNS. Prog. Neurobiol. 83, 2–23 (2007).
Hatakeyama, J., Tomita, K., Inoue, T. & Kageyama, R. Roles of homeobox and bHLH genes in specification of a retinal cell type. Development 128, 1313–1322 (2001).
Zhao, C., Teng, E.M., Summers, R.G., Jr., Ming, G.L. & Gage, F.H. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J. Neurosci. 26, 3–11 (2006).
Vierbuchen, T. et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 3, 1035–1041 (2010).
Zhou, Q. & Melton, D.A. Extreme makeover: converting one cell into another. Cell Stem Cell 3, 382–388 (2008).
Glaser, T. et al. Adult germ line stem cells as a source of functional neurons and glia. Stem Cells 26, 2434–2443 (2008).
Varga, B.V. et al. Generation of diverse neuronal subtypes in cloned populations of stem-like cells. BMC Dev. Biol. 8, 89 (2008).
Berninger, B., Guillemot, F. & Götz, M. Directing neurotransmitter identity of neurones derived from expanded adult neural stem cells. Eur. J. Neurosci. 25, 2581–2590 (2007).
Reyes, J.H. et al. Glutamatergic neuronal differentiation of mouse embryonic stem cells after transient expression of neurogenin 1 and treatment with BDNF and GDNF: in vitro and in vivo studies. J. Neurosci. 28, 12622–12631 (2008).
Gaspard, N. et al. Generation of cortical neurons from mouse embryonic stem cells. Nat. Protoc. 4, 1454–1463 (2009).
Bibel, M. et al. Differentiation of mouse embryonic stem cells into a defined neuronal lineage. Nat. Neurosci. 7, 1003–1009 (2004).
Bibel, M., Richter, J., Lacroix, E. & Barde, Y.A. Generation of a defined and uniform population of CNS progenitors and neurons from mouse embryonic stem cells. Nat. Protoc. 2, 1034–1043 (2007).
Chatzi, C. et al. Derivation of homogeneous GABAergic neurons from mouse embryonic stem cells. Exp. Neurol. 217, 407–416 (2009).
Watanabe, K. et al. Directed differentiation of telencephalic precursors from embryonic stem cells. Nat. Neurosci. 8, 288–296 (2005).
Gaspard, N. et al. An intrinsic mechanism of corticogenesis from embryonic stem cells. Nature 455, 351–357 (2008).
Shen, Q. et al. The timing of cortical neurogenesis is encoded within lineages of individual progenitor cells. Nat. Neurosci. 9, 743–751 (2006).
Nikoletopoulou, V. et al. Neurotrophin receptor-mediated death of misspecified neurons generated from embryonic stem cells lacking Pax6. Cell Stem Cell 1, 529–540 (2007).
Dragunow, M. The adult human brain in preclinical drug development. Nat. Rev. Drug Discov. 7, 659–666 (2008).
Buffo, A. et al. Origin and progeny of reactive gliosis: a source of multipotent cells in the injured brain. Proc. Natl Acad. Sci. USA 105, 3581–3586 (2008).
Laywell, E.D., Rakic, P., Kukekov, V.G., Holland, E.C. & Steindler, D.A. Identification of a multipotent astrocytic stem cell in the immature and adult mouse brain. Proc. Natl. Acad. Sci. USA 97, 13883–13888 (2000).
Blum, R. et al. Neuronal network formation from reprogrammed early postnatal rat cortical glial cells. Cereb. Cortex 21, 413–424 (2011).
Naviaux, R.K., Costanzi, E., Haas, M. & Verma, I.M. The pCL vector system: rapid production of helper-free, high-titer, recombinant retroviruses. J. Virol. 70, 5701–5705 (1996).
Tashiro, A., Zhao, C. & Gage, F.H. Retrovirus-mediated single-cell gene knockout technique in adult newborn neurons in vivo. Nat. Protoc. 1, 3049–3055 (2006).
Mori, T. et al. Inducible gene deletion in astroglia and radial glia—a valuable tool for functional and lineage analysis. Glia 54, 21–34 (2006).
Novak, A., Guo, C., Yang, W., Nagy, A. & Lobe, C.G. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis 28, 147–155 (2000).
Brewer, G.J. & Cotman, C.W. Survival and growth of hippocampal neurons in defined medium at low density: advantages of a sandwich culture technique or low oxygen. Brain Res. 494, 65–74 (1989).
Brill, M.S. et al. A dlx2- and pax6-dependent transcriptional code for periglomerular neuron specification in the adult olfactory bulb. J. Neurosci. 28, 6439–6452 (2008).
Ory, D.S., Neugeboren, B.A. & Mulligan, R.C. A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc. Natl. Acad. Sci. USA 93, 11400–11406 (1996).
Giorgetti, A. et al. Generation of induced pluripotent stem cells from human cord blood cells with only two factors: Oct4 and Sox2. Nat. Protoc. 5, 811–820 (2010).
Rieger, M.A., Hoppe, P.S., Smejkal, B.M., Eitelhuber, A.C. & Schroeder, T. Hematopoietic cytokines can instruct lineage choice. Science 325, 217–218 (2009).
Johansson, C.B., Svensson, M., Wallstedt, L., Janson, A.M. & Frisen, J. Neural stem cells in the adult human brain. Exp. Cell Res. 253, 733–736 (1999).
Fitzsimonds, R.M., Song, H.J. & Poo, M.M. Propagation of activity-dependent synaptic depression in simple neural networks. Nature 388, 439–448 (1997).
Schinder, A.F., Berninger, B. & Poo, M. Postsynaptic target specificity of neurotrophin-induced presynaptic potentiation. Neuron 25, 151–163 (2000).
Cummins, T.R., Rush, A.M., Estacion, M., Dib-Hajj, S.D. & Waxman, S.G. Voltage-clamp and current-clamp recordings from mammalian DRG neurons. Nat. Protoc. 4, 1103–1112 (2009).
Lippiat, J.D. Whole-cell recording using the perforated patch clamp technique. Methods Mol. Biol. 491, 141–149 (2008).
Bekkers, J.M. & Stevens, C.F. Excitatory and inhibitory autaptic currents in isolated hippocampal neurons maintained in cell culture. Proc. Natl. Acad. Sci. USA 88, 7834–7838 (1991).
Acknowledgements
This work was supported by grants from the Deutsche Forschungsgemeinschaft to B.B. and M.G. (BE 4182/1-3 and GO 640/9-1) and to T.S.-E. (SCHR 1142/1-1), and by the Bavarian State Ministry of Sciences, Research and the Arts (ForNeuroCell) to B.B. and M.G. We thank S. Bauer for virus production.
Author information
Authors and Affiliations
Contributions
C.H. contributed to protocol design, generation and characterization of the astroglia-derived neurons and preparation of the manuscript; S.G. to the design of the transfection procedure and single-cell tracking by time-lapse video microscopy; G.M. to the characterization of the astroglia-derived neurons; A.L. and R.S. to viral vector design; T.S.-E. to astroglia culture and immunocytochemistry; and T.S. to single-cell tracking by time-lapse video microscopy. M.G. pioneered this protocol for astroglia-to-neuron conversion and contributed to the current protocol design and the manuscript; B.B. contributed to protocol design, electrophysiological characterization of the astroglia-derived neurons and preparation of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Video 1
Direct visualisation of the astroglia-to-neuron metamorphosis. (AVI 8843 kb)
Temporal sequence of astroglia-to-neuron conversion by Neurog2 of a fate-mapped astroglia derived from GLAST::CreERT2/Z/EG mice following recombination in vivo between P2-P7. The movie starts with a GFP-fluorescence and bright field image at the beginning of the time-lapse experiment. The GFP-positive cell is marked with a red arrow throughout the sequence. The cell is subsequently followed using bright field images. At the point when DsRed fluorescence is detectable, a GFP fluorescence image is shown to demonstrate the co-expression of DsRed and GFP in the very same cell. Subsequently, DsRed fluorescence images (grey scale) monitor the transduced cell and its gradual metamorphosis into a neuron. At the end of the imaging sequence a GFP fluorescence image is shown to confirm that the neuron is indeed GFP reporter-positive. The time is indicated in each image. For details on the single-cell tracking method see Rieger et al. (2009).
References
Rieger, M. A., Hoppe, P. S., Smejkal, B. M., Eitelhuber, A. C. & Schroeder, T. Hematopoietic cytokines can instruct lineage choice. Science 325, 217–218 (2009).
Rights and permissions
About this article
Cite this article
Heinrich, C., Gascón, S., Masserdotti, G. et al. Generation of subtype-specific neurons from postnatal astroglia of the mouse cerebral cortex. Nat Protoc 6, 214–228 (2011). https://doi.org/10.1038/nprot.2010.188
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2010.188
This article is cited by
-
The atypical Rho GTPase Rnd2 is critical for dentate granule neuron development and anxiety-like behavior during adult but not neonatal neurogenesis
Molecular Psychiatry (2021)
-
Proneural factors Ascl1 and Neurog2 contribute to neuronal subtype identities by establishing distinct chromatin landscapes
Nature Neuroscience (2019)
-
Transdifferentiation: a new promise for neurodegenerative diseases
Cell Death & Disease (2018)
-
Engineering new neurons: in vivo reprogramming in mammalian brain and spinal cord
Cell and Tissue Research (2018)
-
Induction of functional dopamine neurons from human astrocytes in vitro and mouse astrocytes in a Parkinson's disease model
Nature Biotechnology (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.