Abstract
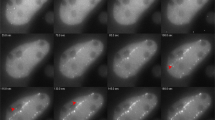
We describe a protocol for creating localized DNA double-strand breaks (DSBs) with minimal requirements that can be applied in cell biology and molecular biology. This protocol is based on the combination of 5-bromo-2′-deoxyuridine (BrdU) labeling and ultraviolet C (UVC) irradiation through porous membranes. Cells are labeled with 10 μM BrdU for 48–72 h, washed with Ca2+- and Mg2+-free PBS(–), covered by polycarbonate membranes with micropores and exposed to UVC light. With this protocol, localized DSBs are created within subnuclear areas, irrespective of the cell cycle phase. Recruitment of proteins involved in DNA repair, DNA damage response, chromatin remodeling and histone modifications can be visualized without any specialized equipment. The quality is the same as that obtained by laser microirradiation or by any other focal irradiation. DSBs become visible within 30 min of UVC irradiation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Paull, T.T. et al. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr. Biol. 10, 886–895 (2000).
Bonner, W.M. et al. GammaH2AX and cancer. Nat. Rev. Cancer 8, 957–967 (2008).
Lukas, C., Bartek, J. & Lukas, J. Imaging of protein movement induced by chromosomal breakage: tiny 'local' lesions pose great 'global' challenges. Chromosoma 114, 146–154 (2005).
Stucki, M. & Jackson, S.P. gammaH2AX and MDC1: anchoring the DNA-damage-response machinery to broken chromosomes. DNA Repair 5, 534–543 (2006).
Kinner, A., Wu, W., Staudt, C. & Iliakis, G. Gamma-H2AX in recognition and signalling of DNA double-strand breaks in the context of chromatin. Nucleic Acids Res. 36, 5678–5694 (2008).
FitzGerald, J.E., Grenon, M. & Lowndes, N.F. 53BP1: function and mechanism of focal recruitment. Biochem. Soc. Trans. 37, 897–904 (2009).
Misteli, T. & Soutoglou, E. The emerging role of nuclear architecture in DNA repair and genome maintenance. Nat. Rev. Mol. Cell Biol. 10, 243–254 (2009).
Bekker-Jensen, S. et al. Spatial organization of the mammalian genome surveillance machinery in response to DNA strand breaks. J. Cell Biol. 173, 195–206 (2006).
Chan, D.W. et al. Autophosphorylation of the DNA-dependent protein kinase catalytic subunit is required for rejoining of DNA double-strand breaks. Genes Dev. 16, 2333–2338 (2002).
Chen, B.P.C. et al. Cell cycle dependence of DNA-dependent protein kinase phosphorylation in response to DNA double strand breaks. J. Biol. Chem. 280, 14709–14715 (2005).
Rogakou, E.P., Boon, C., Redon, C. & Bonner, W.M. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J. Cell Biol. 146, 905–915 (1999).
Tashiro, S. et al. Rad51 accumulation at sites of DNA damage and in postreplicative chromatin. J. Cell Biol. 150, 283–291 (2000).
Lukas, C. et al. Distinct spatiotemporal dynamics of mammalian checkpoint regulators induced by DNA damage. Nat. Cell Biol. 5, 255–260 (2003).
Lan, L. et al. In situ analysis of repair processes for oxidative DNA damage in mammalian cells. Proc. Natl. Acad. Sci. USA 101, 13738–13743 (2004).
Kim, J.-S. et al. Specific recruitment of human cohesin to laser-induced DNA damage. J. Biol. Chem. 277, 45149–45153 (2002).
Meldrum, R.A., Botchway, S.W., Wharton, C.W. & Hirst, G.J. Nanoscale spatial induction of ultraviolet photoproducts in cellular DNA by three-photon near-infrared absorption. EMBO J. 12, 1144–1149 (2003).
Dinant, C. et al. Activation of multiple DNA repair pathways by subnuclear damage induction methods. J. Cell Sci. 120, 2731–2740 (2007).
Nagy, Z. & Soutoglou, E. DNA repair: easy to visualize, difficult to elucidate. Trends Cell Biol. 19, 617–629 (2009).
Nelms, B.E., Maser, R.S., Mackay, J.F., Lagally, M.G. & Petrini, J.H.J. In situ visualization of DNA double-strand break repair in human fibroblasts. Science 280, 590–592 (1998).
Tartier, L. et al. Local DNA damage by proton microbeam irradiation induces poly(ADP-ribose) synthesis in mammalian cells. Mutagenesis 18, 411–416 (2003).
Aten, J.A. et al. Dynamics of DNA double-strand breaks revealed by clustering of damaged chromosome domains. Science 303, 92–95 (2004).
Hauptner, A. et al. DNA-repair protein distribution along the tracks of energetic ions. Radiat. Prot. Diodosimetry 122, 147–149 (2006).
Stap, J. et al. Induction of linear tracks of DNA double-strand breaks by alpha-particle irradiation of cells. Nat. Methods 5, 261–266 (2008).
Jakob, B., Splinter, J., Durante, M. & Taucher-Scholz, G. Live cell microscopy analysis of radiation-induced DNA double-strand break motion. Proc. Natl. Acad. Sci. USA 106, 3172–3177 (2009).
van Oven, C. et al. An ultrasoft X-ray multi-microbeam irradiation system for studies of DNA damage responses by fixed-and live-cell fluorescence microscopy. Eur. Biophys. J. 38, 721–728 (2009).
Suzuki, K. et al. A novel and simple micro-irradiation technique for creating localized DNA double-strand breaks. Nucleic Acids Res. 38, e129 (2010).
Katsumi, S. et al. In situ visualization of ultraviolet-light-induced DNA damage repair in locally irradiated human fibroblasts. J. Invest. Dermatol. 117, 1156–1161 (2001).
Moné, M.J. et al. Local UV-induced DNA damage in cell nuclei results in local transcription inhibition. EMBO Rep. 2, 1013–1017 (2001).
Volker, M. et al. Sequential assembly of the nucleotide excision repair factors in vivo. Mol. Cell 8, 213–224 (2001).
Limoli, C.L. & Ward, J.F. A new method for introducing double-strand breaks into cellular DNA. Radiat. Res. 134, 160–169 (1993).
Krasin, F. & Hutchinson, F. Double-strand breaks from single photochemical events in DNA containing 5-bromouracil. Biophys. J. 24, 645–656 (1978).
Marti, T.M., Hefner, E., Feeney, L., Natale, V. & Cleaver, J.E. H2AX phosphorylation within the G1 phase after UV irradiation depends on nucleotide excision repair and not DNA double-strand breaks. Proc. Natl. Acad. Sci. USA 103, 9891–9896 (2006).
Marteijn, J.A. et al. Nucleotide excision repair-induced H2A ubiquitination is dependent on MDC1 and RNF8 and reveals a universal DNA damage response. J. Cell Biol. 186, 835–847 (2009).
Matsumoto, M. et al. Perturbed gap-filling synthesis in nucleotide excision repair causes histone H2AX phosphorylation in human quiescent cells. J. Cell Sci. 120, 1104–1112 (2007).
Acknowledgements
We thank W.F. Morgan for his critical reading of the manuscript and for helpful comments. This study was supported in part by the Global Center of Excellence (GCOE) Program from the Japanese Ministry of Education, Culture, Sports, Science and Technology.
AUTHOR CONTRIBUTIONS
K.S. conceived, designed and conducted the study, and drafted the manuscript. M.Y., Y.O. and M.S. provided reagents, carried out the immunofluorescence study and edited the manuscript. S.Y. supervised the project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Suzuki, K., Yamauchi, M., Oka, Y. et al. Creating localized DNA double-strand breaks with microirradiation. Nat Protoc 6, 134–139 (2011). https://doi.org/10.1038/nprot.2010.183
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2010.183
This article is cited by
-
Deletion of the KU70 homologue facilitates gene targeting in Lipomyces starkeyi strain NRRL Y-11558
Current Genetics (2019)
-
Cellular Depletion of BRD8 Causes p53-Dependent Apoptosis and Induces a DNA Damage Response in Non-Stressed Cells
Scientific Reports (2018)
-
Non-canonical reader modules of BAZ1A promote recovery from DNA damage
Nature Communications (2017)
-
PC4 promotes genome stability and DNA repair through binding of ssDNA at DNA damage sites
Oncogene (2016)
-
Microirradiation techniques in radiobiological research
Journal of Biosciences (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.