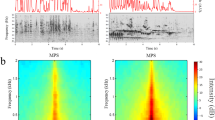
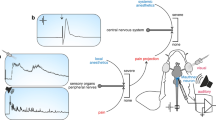
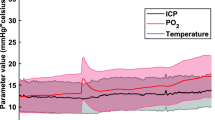
Abstract
Here we describe a protocol for bilateral multielectrode neurophysiological recordings during intracerebral pharmacological manipulations in awake songbirds. This protocol encompasses fitting adult animals with head-posts and recording chambers, and acclimating them to periods of restraint. The adaptation period is followed by bilateral penetrations of multiple electrodes to obtain acute, sensory-driven neurophysiological responses before versus during the application of pharmacological agents of interest. These local manipulations are achieved by simultaneous and restricted drug infusions carried out independently for each hemisphere. We have used this protocol to elucidate how neurotransmitter and neuroendocrine systems shape the auditory and perceptual processing of natural, learned communication signals. However, this protocol can be used to explore the neurochemical basis of sensory processing in other small vertebrates. Representative results and troubleshooting of key steps of this protocol are presented. Following the animal's recovery from head-post and recording chamber implantation surgery, the length of the procedure is 2 d.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Rinberg, D., Koulakov, A. & Gelperin, A. Sparse odor coding in awake behaving mice. J. Neurosci. 26, 8857–8865 (2006).
Georgopoulos, A.P., Merchant, H., Naselaris, T. & Amirikian, B. Mapping of the preferred direction in the motor cortex. Proc. Natl. Acad. Sci. USA 104, 11068–11072 (2007).
Sillito, A.M. The contribution of inhibitory mechanisms to the receptive field properties of neurones in the striate cortex of the cat. J. Physiol. (Lond.) 250, 305–329 (1975).
Hicks, T.P., Albus, K., Kaneko, T. & Baumfalk, U. Examination of the effects of cholecystokinin 26–33 and neuropeptide Y on responses of visual cortical neurons of the cat. Neuroscience 52, 263–279 (1993).
Tremere, L., Hicks, T.P. & Rasmusson, D.D. Role of inhibition in cortical reorganization of the adult raccoon revealed by microiontophoretic blockade of GABA(A) receptors. J. Neurophysiol. 86, 94–103 (2001).
Hubel, D.H. & Wiesel, T.N. Receptive fields and functional architecture in two nonstriate visual areas (18 and 19) of the cat. J. Neurophysiol. 28, 229–289 (1965).
Hubel, D.H. & Wiesel, T.N. Receptive fields and functional architecture of monkey striate cortex. J. Physiol. (Lond.) 195, 215–243 (1968).
Suga, N., Zhang, Y. & Yan, J. Sharpening of frequency tuning by inhibition in the thalamic auditory nucleus of the mustached bat. J. Neurophysiol. 77, 2098–2114 (1997).
Miller, E.K. & Wilson, M.A. All my circuits: using multiple electrodes to understand functioning neural networks. Neuron 60, 483–488 (2008).
Platt, M.L. & Huettel, S.A. Risky business: the neuroeconomics of decision making under uncertainty. Nat. Neurosci. 11, 398–403 (2008).
Woolley, S.M., Gill, P.R., Fremouw, T. & Theunissen, F.E. Functional groups in the avian auditory system. J. Neurosci. 29, 2780–2793 (2009).
Gentner, T.Q. & Margoliash, D. Neuronal populations and single cells representing learned auditory objects. Nature 424, 669–674 (2003).
Schmidt, M.F. & Konishi, M. Gating of auditory responses in the vocal control system of awake songbirds. Nat. Neurosci. 1, 513–518 (1998).
Huetz, C., Philibert, B. & Edeline, J.M. A spike-timing code for discriminating conspecific vocalizations in the thalamocortical system of anesthetized and awake guinea pigs. J. Neurosci. 29, 334–350 (2009).
Mouly, A.M., Fort, A., Ben-Boutayab, N. & Gervais, R. Olfactory learning induces differential long-lasting changes in rat central olfactory pathways. Neuroscience 102, 11–21 (2001).
Olivéras, J.L., Montagne-Clavel, J. & Martin, G. Drastic changes of ventromedial medulla neuronal properties induced by barbiturate anesthesia. I. Comparison of the single-unit types in the same awake and pentobarbital-treated rats. Brain Res. 563, 241–250 (1991).
Supèr, H. & Roelfsema, P.R. Chronic multiunit recordings in behaving animals: advantages and limitations. Prog. Brain Res. 147, 263–282 (2005).
Chiu, C. & Weliky, M. Multi-electrode recording from the developing visual pathway of awake behaving ferrets. J. Neurosci. Methods 136, 55–61 (2004).
Tolias, A.S. et al. Recording chronically from the same neurons in awake, behaving primates. J. Neurophysiol. 98, 3780–3790 (2007).
Crandall, S.R., Adam, M., Kinnischtzke, A.K. & Nick, T.A. HVC neural sleep activity increases with development and parallels nightly changes in song behavior. J. Neurophysiol. 98, 232–240 (2007).
Crandall, S.R., Aoki, N. & Nick, T.A. Developmental modulation of the temporal relationship between brain and behavior. J. Neurophysiol. 97, 806–816 (2007).
McCasland, J.S. & Konishi, M. Interaction between auditory and motor activities in an avian song control nucleus. Proc. Natl. Acad. Sci. USA 78, 7815–7819 (1981).
Stripling, R., Volman, S.F. & Clayton, D.F. Response modulation in the zebra finch neostriatum: relationship to nuclear gene regulation. J. Neurosci. 17, 3883–3893 (1997).
Doupe, A.J. & Konishi, M. Song-selective auditory circuits in the vocal control system of the zebra finch. Proc. Natl. Acad. Sci. USA 88, 11339–11343 (1991).
Stripling, R., Kruse, A.A. & Clayton, D.F. Development of song responses in the zebra finch caudomedial neostriatum: role of genomic and electrophysiological activities. J. Neurobiol. 48, 163–180 (2001).
Hessler, N.A. & Doupe, A.J. Singing-related neural activity in a dorsal forebrain-basal ganglia circuit of adult zebra finches. J. Neurosci. 19, 10461–10481 (1999).
Capsius, B. & Leppelsack, H.J. Influence of urethane anesthesia on neural processing in the auditory cortex analogue of a songbird. Hear. Res. 96, 59–70 (1996).
Gehr, D.D., Hofer, S.B., Marquardt, D. & Leppelsack, H. Functional changes in field L complex during song development of juvenile male zebra finches. Brain Res. Dev. Brain Res. 125, 153–165 (2000).
Chew, S.J., Mello, C., Nottebohm, F., Jarvis, E. & Vicario, D.S. Decrements in auditory responses to a repeated conspecific song are long-lasting and require two periods of protein synthesis in the songbird forebrain. Proc. Natl. Acad. Sci. USA 92, 3406–3410 (1995).
Chew, S.J., Vicario, D.S. & Nottebohm, F. A large-capacity memory system that recognizes the calls and songs of individual birds. Proc. Natl. Acad. Sci. USA 93, 1950–1955 (1996).
Vicario, D.S. & Yohay, K.H. Song-selective auditory input to a forebrain vocal control nucleus in the zebra finch. J. Neurobiol. 24, 488–505 (1993).
Pinaud, R. et al. Inhibitory network interactions shape the auditory processing of natural communication signals in the songbird auditory forebrain. J. Neurophysiol. 100, 441–455 (2008).
Vicario, D.S. & Raksin, J.N. Possible roles for GABAergic inhibition in the vocal control system of the zebra finch. Neuroreport 11, 3631–3635 (2000).
Rauske, P.L., Shea, S.D. & Margoliash, D. State and neuronal class-dependent reconfiguration in the avian song system. J. Neurophysiol. 89, 1688–1701 (2003).
Dave, A.S., Yu, A.C. & Margoliash, D. Behavioral state modulation of auditory activity in a vocal motor system. Science 282, 2250–2254 (1998).
George, I., Cousillas, H., Richard, J.P. & Hausberger, M. A new extensive approach to single unit responses using multisite recording electrodes: application to the songbird brain. J. Neurosci. Methods 125, 65–71 (2003).
Nick, T.A. & Konishi, M. Dynamic control of auditory activity during sleep: correlation between song response and EEG. Proc. Natl. Acad. Sci. USA 98, 14012–14016 (2001).
Müller, C.M. & Scheich, H. Contribution of GABAergic inhibition to the response characteristics of auditory units in the avian forebrain. J. Neurophysiol. 59, 1673–1689 (1988).
Terleph, T.A., Lu, K. & Vicario, D.S. Response properties of the auditory telencephalon in songbirds change with recent experience and season. PLoS One 3, e2854 (2008).
Terleph, T.A., Mello, C.V. & Vicario, D.S. Auditory topography and temporal response dynamics of canary caudal telencephalon. J. Neurobiol. 66, 281–292 (2006).
Terleph, T.A., Mello, C.V. & Vicario, D.S. Species differences in auditory processing dynamics in songbird auditory telencephalon. Dev. Neurobiol. 67, 1498–1510 (2007).
Tremere, L.A., Jeong, J.K. & Pinaud, R. Estradiol shapes auditory processing in the adult brain by regulating inhibitory transmission and plasticity-associated gene expression. J. Neurosci. 29, 5949–5963 (2009).
Hofer, S.B. & Klump, G.M. Within- and across-channel processing in auditory masking: a physiological study in the songbird forebrain. J. Neurosci. 23, 5732–5739 (2003).
Acknowledgements
This study was supported by the NIH/NIDCD and start-up packages from the University of Rochester to R.P. and L.A.T. We thank M. Weliky for insightful discussions and the three anonymous reviewers for constructive feedback. The authors are especially indebted to E. Jarvis for providing the conditions and support necessary for the development of the multielectrode array described here. We also thank D. Vicario in whose laboratory two of us (T.T. and R.P.) were exposed to basic concepts and techniques that provided the framework for the advancement of this methodology.
Author information
Authors and Affiliations
Contributions
L.A.T., T.A.T. and R.P. extended the method; L.A.T., J.K.J. and R.P. refined the method; L.A.T. customized the stereotaxic equipment and collected the data presented in the paper; L.A.T., T.A.T., J.K.J. and R.P. wrote the paper.
Corresponding authors
Rights and permissions
About this article
Cite this article
Tremere, L., Terleph, T., Jeong, J. et al. Bilateral multielectrode neurophysiological recordings coupled to local pharmacology in awake songbirds. Nat Protoc 5, 191–200 (2010). https://doi.org/10.1038/nprot.2009.224
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2009.224
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.